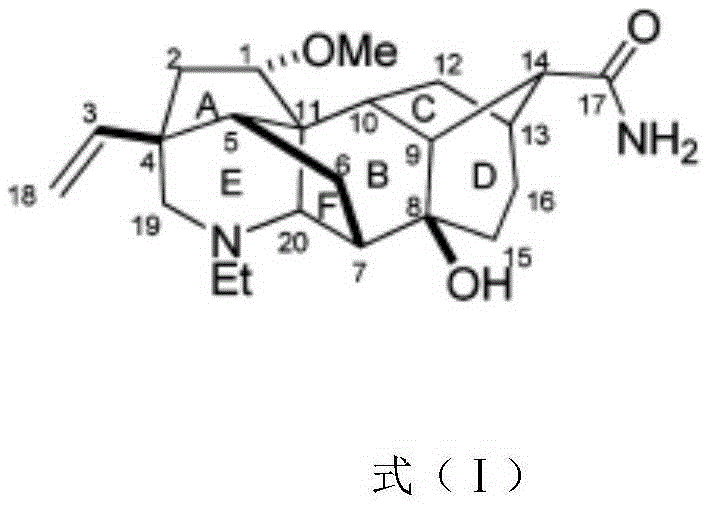
Application of Racemulsonine in preparing medicine for resisting human body fungi
An antifungal and drug technology, applied in the direction of antifungal agents, can solve the problems of irritation, dizziness, allergy, strong accumulation of toxicity, etc., and achieve the effect of outstanding substantive characteristics and strong inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] Embodiment 1: the preparation of compound Racemulsonine tablet involved in the present invention:
[0011] Take 5 grams of the compound Racemulsonine, add 195 grams of dextrin, mix well, and make 1000 tablets by conventional compression.
Embodiment 2
[0012] Embodiment 2: the preparation of compound Racemulsonine capsules involved in the present invention:
[0013] Take 5 grams of the compound Racemulsonine, add 195 grams of starch, mix well, and make 1000 capsules.
[0014] The following pharmacodynamic experiments will further illustrate its drug activity.
experiment example 1
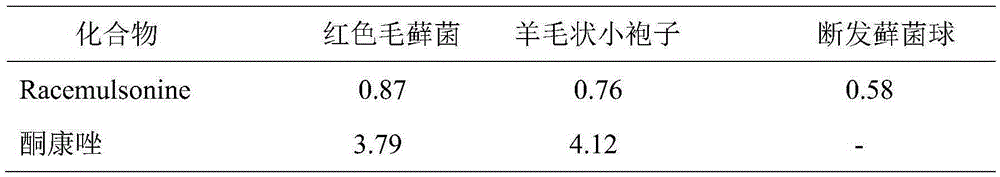
[0015] Experimental example 1: Racemulsonine anti-fungal activity in humans
[0016] The anti-fungal activity experiment against human body adopts the method of concentration dilution, and each determination is repeated three times. The test pathogens include Trichomonas rubrum, Microsporum lanoides and Trichomonas clumps, and the concentration of bacteria solution is 105 / mL. The initial concentration of Racemulsonine is 50.0 μg / mL (5% dimethyl sulfoxide DMSO), serially diluted to 0.098 μg / mL, and the equal volume of bacterial liquid and test samples are mixed and cultured in a 96-well plate, and the culture temperature of human fungi is respectively 28°C, observe after 24 hours of incubation time, if no colonies are found, it is the lowest anti-human fungal concentration of the sample, that is, the MIC value. The positive control of this experiment is ketoconazole, and the results of Racemulsonine against human fungi are shown in Table 1.
[0017] Table 1 Racemulsonine anti-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com