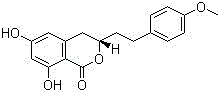
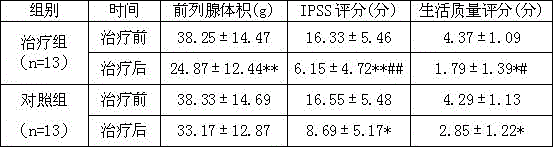
Medicine composition for treating prostatic hyperplasia
A technology of prostatic hyperplasia and composition, which is applied in the direction of drug combination, medical formula, plant raw materials, etc., can solve the problems of different syndrome types, diagnostic criteria, curative effect criteria, case selection criteria, unfavorable screening of effective prescriptions and drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the pharmaceutical composition for the treatment of benign prostatic hyperplasia and preparation method thereof
[0037] The composition and parts by weight of the crude drug of the pharmaceutical composition for treating benign prostatic hyperplasia are: Tongan Lengshuihua 3085g Ailei 2225g Hydroxyevodiamine 167g Lepidolin C213g Agrimony lactone 190g;
[0038] Preparation:
[0039] (1) According to the ratio of the raw materials, take the cold water flower of the stem, Elephant, hydroxyevodiamine, liquiritigenin C, and agrimony lactone, mix them evenly, use ethanol with a concentration of 23% by weight as a solvent, and soak at 22°C Extraction, the number of times of extraction is 17 times, each extraction time is 37 hours, each solvent consumption is 31 times of the total weight of the raw material drug, and it is filtered to obtain medicinal residue A and extract A, and the extract A reclaims ethanol and concentrates to a relative Density 1.06, filter,...
Embodiment 2
[0042] Embodiment 2: the pharmaceutical composition for the treatment of benign prostatic hyperplasia and preparation method thereof
[0043] The composition and parts by weight of the raw material drug of the pharmaceutical composition for treating benign prostatic hyperplasia are: 3080g of Tongan Lengshuihua, 2230g of hydroxyevodiamine, 165g of evodiamine C216g, and 180g of Agrimony lactone;
[0044] Preparation:
[0045] (1) According to the ratio of the raw materials, take the cold water flower of the stem, Elephant, hydroxyevodiamine, liquiritigenin C, and agrimony lactone, mix them evenly, use ethanol with a concentration of 23% by weight as a solvent, and soak at 22°C Extraction, the number of times of extraction is 17 times, each extraction time is 37 hours, each solvent consumption is 31 times of the total weight of the raw material drug, and it is filtered to obtain medicinal residue A and extract A, and the extract A reclaims ethanol and concentrates to a relative ...
Embodiment 3
[0048] Embodiment 3: the pharmaceutical composition for the treatment of benign prostatic hyperplasia and preparation method thereof
[0049] The composition and parts by weight of the crude drug of the pharmaceutical composition for treating benign prostatic hyperplasia are: Tongan Lengshuihua 3090g Ailei 2220g Hydroxyevodiamine 170g Lepidolin C210g Agrimony lactone 200g;
[0050] Preparation:
[0051] (1) According to the ratio of the raw materials, take the cold water flower of the stem, Elephant, hydroxyevodiamine, liquiritigenin C, and agrimony lactone, mix them evenly, use ethanol with a concentration of 23% by weight as a solvent, and soak at 22°C Extraction, the number of times of extraction is 17 times, each extraction time is 37 hours, each solvent consumption is 31 times of the total weight of the raw material drug, and it is filtered to obtain medicinal residue A and extract A, and the extract A reclaims ethanol and concentrates to a relative Density 1.06, filter,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com