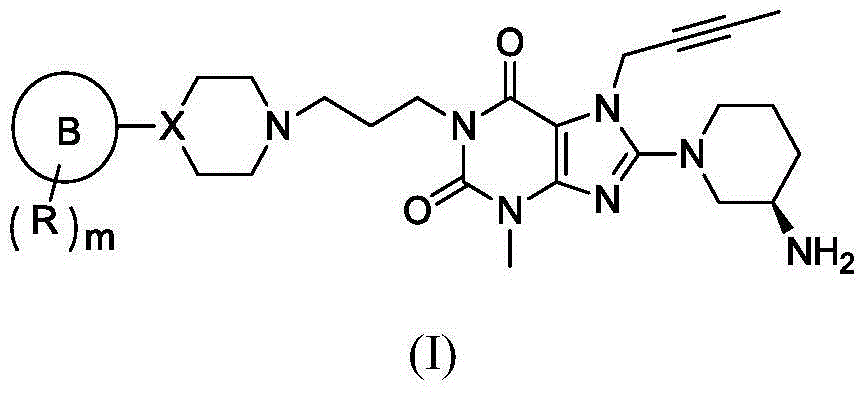
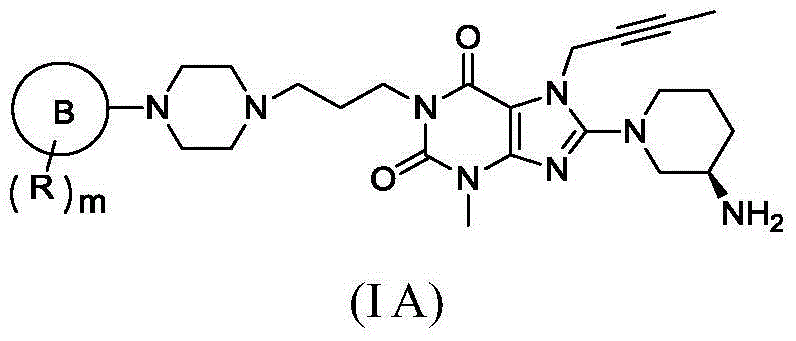
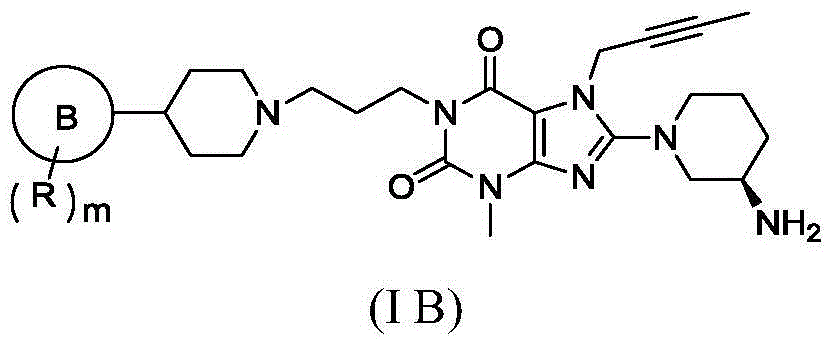
Five-membered-aromatic-heterocycte-containing substituted xanthine compound and preparation method and use thereof
A compound, heterocycloalkyl technology, applied in the field of medicine, can solve the problem of short half-life of active GLP-1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0211] Synthetic route of route 1 intermediate 3a-1
[0212]
[0213] The first step 8-[(3R)-3-(tert-butoxycarbonyl)aminopiperidin-1-yl]-7-(2-butynyl)-3,7-dihydro-3-methyl-1H - Preparation of purine-2,6-dione 2a
[0214] 8-Bromo-7-(2-butynyl)-3,7-dihydro-3-methyl-1H-purine-2,6-dione 1a (2.08g, 7.00mmol), (R)- 3-(tert-butoxycarbonyl)aminopiperidine (1.75g, 8.75mmol) and potassium carbonate (1.94g, 14.00mmol) were placed in a 50mL single-necked bottle, DMF (15mL) was added, and reacted at 75°C under the protection of argon After 7 hours, TLC detected that the reaction was complete. DMF was evaporated to obtain a tan solid, which was slurried by adding 50 mL of water, filtered, and dried under infrared light to obtain 2a, 2.60 g of a tan solid, with a yield of 89.7%. 1 HNMR (400MHz, CDCl 3 )δ:7.84(brs,1H),5.60(brs,1H),4.85(m,2H),3.86(m,1H),3.54(m,1H),3.53(s,3H),3.36(m,2H ),3.28(m,1H),1.89(m,2H),1.82(s,3H),1.73(m,2H),1.45(s,9H).
[0215] The second step 8-[(3R)-3-(tert-but...
Embodiment 1
[0379]
[0380] Compound 18-[(3R)-3-aminopiperidin-1-yl]-7-(2-butynyl)-3,7-dihydro-3-methyl-1-{3-[4-( 3-Phenyl-1,2,4-oxadiazol-5-yl)piperazin-1-yl]propyl}-1H-purine-2,6-dione
[0381] method one:
[0382]
[0383] Method Two:
[0384]
[0385] The first step 8-[(3R)-3-(tert-butoxycarbonyl)aminopiperidin-1-yl]-7-(2-butynyl)-3,7-dihydro-3-methyl-1 -{3-[4-(3-Phenyl-1,2,4-oxadiazol-5-yl)piperazin-1-yl]propyl}-1H-purine-2,6-dione 1c preparation
[0386] method one:
[0387] 8-[(3R)-3-(tert-butoxycarbonyl)aminopiperidinyl-1-]-7-(2-butynyl)-3,7-dihydro-3-methyl-1-( 3-bromopropyl)-1H-purine-2,6-dione 3a-1 (0.42g, 0.77mmol) and 1-(3-phenyl-1,2,4-oxadiazol-5-yl) Piperazine 1b (0.18g, 0.78mmol) was dissolved in 3mL of anhydrous DMF, then DIPEA (0.40mL, 2.32mmol) was added, and reacted at 75°C for 8h under the protection of argon. It was detected by TLC that the reaction of the raw material was complete, the solvent was evaporated, 20 mL of deionized water was added, extra...
Embodiment 2
[0394]
[0395]Compound 28-[(3R)-3-aminopiperidin-1-yl]-7-(2-butynyl)-3,7-dihydro-3-methyl-1-{3-[4-( 3-(pyridin-4-yl)-1,2,4-oxadiazol-5-yl)piperazin-1-yl]propyl}-1H-purine-2,6-dione
[0396]
[0397] The first step 8-[(3R)-3-(tert-butoxycarbonyl)aminopiperidin-1-yl]-7-(2-butynyl)-3,7-dihydro-3-methyl-1 -{3-[4-(3-(pyridin-4-yl)-1,2,4-oxadiazol-5-yl)piperazin-1-yl]propyl}-1H-purine-2,6 - Preparation of diketone 2c
[0398] Starting from 3a-1 (0.20g, 0.37mmol) and 1-[3-(4-pyridyl)-1,2,4-oxadiazol-5-yl)piperazine 2b (0.09g, 0.39mmol) , using the steps similar to the first step in Example 1, the intermediate 2c was obtained, 0.14 g of white solid, and the yield was 54.7%.
[0399]
[0400] The second step 8-[(3R)-3-aminopiperidin-1-yl]-7-(2-butynyl)-3,7-dihydro-3-methyl-1-{3-[4 Preparation of -(3-(pyridin-4-yl)-1,2,4-oxadiazol-5-yl)piperazin-1-yl]propyl}-1H-purine-2,6-dione 2
[0401] Using 2c (0.13g, 0.19mmol) as the starting material, the second step in Example 1 wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com