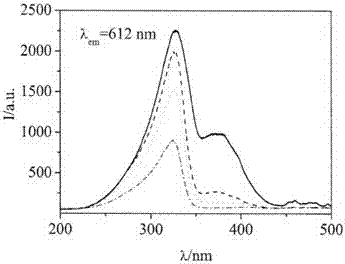
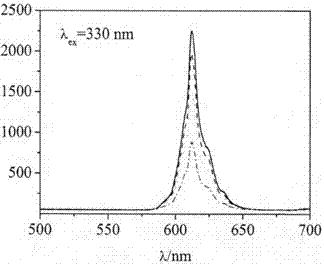
A kind of red long afterglow material and preparation method thereof
A long afterglow material, red technology, applied in the direction of luminescent materials, chemical instruments and methods, etc., can solve the problems of long afterglow time, inconsistent energy consumption, etc., and achieve the effect of long afterglow time, uniform distribution and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Measure Ca salt solution, Zn salt solution, or Eu according to the stoichiometric ratio of the above chemical formula 3+ salt solution, Al 3+ The salt solution of butyl titanate and the acid solution of butyl titanate are added into the container to form a mixed solution, then the dispersant is added and mixed evenly under the condition of supergravity, the solid and liquid are separated, the solid phase is washed with water, and the washed solid phase is mixed with The organic solvent is mixed evenly to obtain the feed liquid;
[0033] (2) Input the feed liquid obtained in step (1) into the reaction kettle, raise the temperature of the feed liquid to 160~300°C under stirring and keep it at this temperature for 0.5~6h, and then continuously feed and discharge under stirring The crystallization reaction is carried out in the circulation mode to obtain the precursor slurry, the temperature of the crystallization reaction is controlled to be 160~300°C, the residence t...
Embodiment 2
[0036] In this embodiment, the chemical formula of the red long afterglow material is Ca 0.8 Zn 0.2 TiO 3 :Pr 3+ ,Al 3+ ,Mg 2+ , where, Pr 3+ The content is matrix Ca 0.8 Zn 0.2 TiO 3 0.1wt% of Al 3+ and Mg 2+ matrix Ca 0.8 Zn 0.2 TiO 3 0.15wt% and 0.1wt%, its preparation method is as follows:
[0037] (1) Dissolve calcium nitrate in deionized water to prepare 0.2mol / L calcium nitrate aqueous solution; dissolve zinc nitrate in deionized water to prepare 0.5mol / L zinc nitrate aqueous solution; dissolve praseodymium nitrate in deionized water to prepare into 1.0mol / L praseodymium nitrate aqueous solution; dissolving aluminum nitrate in deionized water to prepare 1.0mol / L aluminum nitrate aqueous solution; dissolving magnesium nitrate in deionized water to prepare 1.0mol / L magnesium nitrate aqueous solution; Sodium oxide was dissolved in deionized water to prepare a 200g / L aqueous sodium hydroxide solution. Add butyl titanate to citric acid solution to form an acid...
Embodiment 3
[0043] In this embodiment, the chemical formula of the red long afterglow material is Ca 0.8 Zn 0.2 TiO 3 :Pr 3+ ,Al 3+ ,Mg 2+ , where, Pr 3+ The content is matrix Ca 0.8 Zn 0.2 TiO 3 0.3wt% of Al 3+ and Mg 2+ matrix Ca 0.8 Zn 0.2 TiO 3 1wt% and 0.15wt%, its preparation method is as follows:
[0044] (1) Dissolve calcium nitrate in deionized water to prepare 0.5mol / L calcium nitrate aqueous solution; dissolve zinc nitrate in deionized water to prepare 1.5mol / L zinc nitrate aqueous solution; dissolve praseodymium nitrate in deionized water to prepare into 1.5mol / L praseodymium nitrate aqueous solution; dissolving aluminum nitrate in deionized water to prepare 1.5mol / L aluminum nitrate aqueous solution; dissolving magnesium nitrate in deionized water to prepare 1.0mol / L magnesium nitrate aqueous solution; hydrogen Potassium oxide was dissolved in deionized water to prepare a 100g / L aqueous sodium hydroxide solution. Add butyl titanate to glacial acetic acid soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com