Near-infrared long-afterglow luminescent material having photo-stimulated luminescence and its preparation method and use
A luminescent material and light-excited technology, applied in luminescent materials, chemical instruments and methods, preparations for in vivo experiments, etc., can solve problems such as inability to achieve multiple detections, luminous intensity attenuation, etc., to increase afterglow time, increase Effect of Luminous Intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] According to the following composition: near-infrared long afterglow luminescent material LaAlO 3 :Mn x , wherein the doping amount x of Mn ions is 0.0001mol%; respectively weigh lanthanum oxide, aluminum oxide, and manganese carbonate, grind and mix them, pre-fire at 600°C for 4 hours, take them out, grind again, and fire at 1300°C 5 hours.
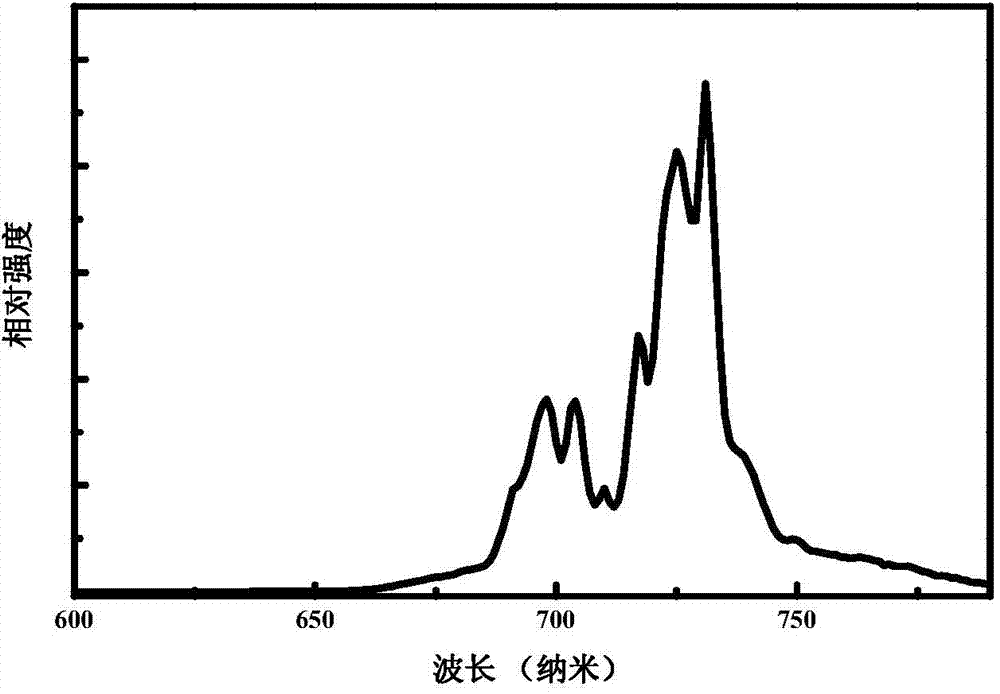
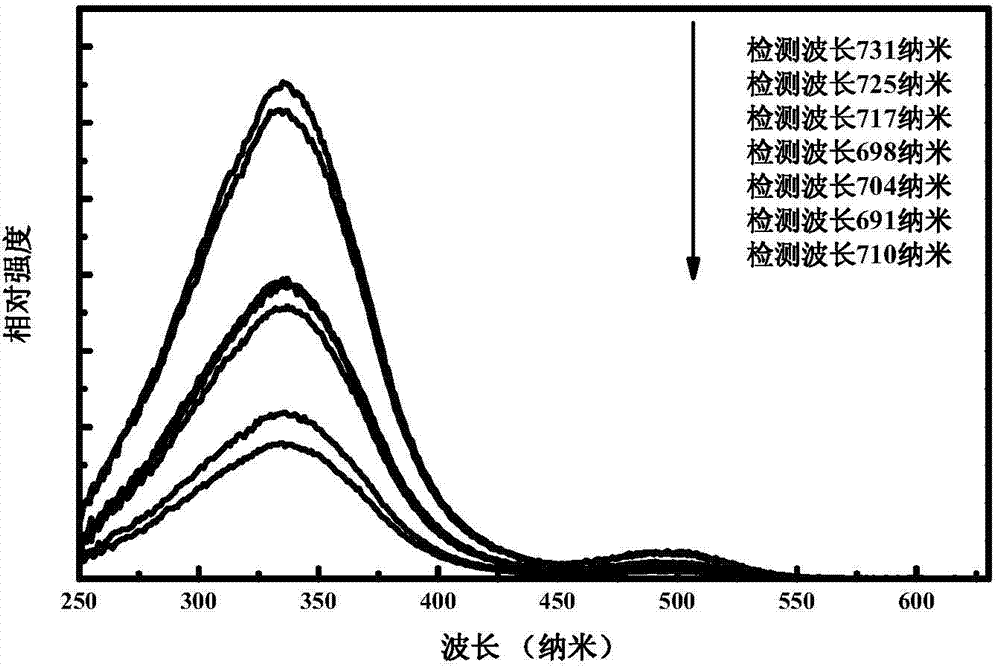
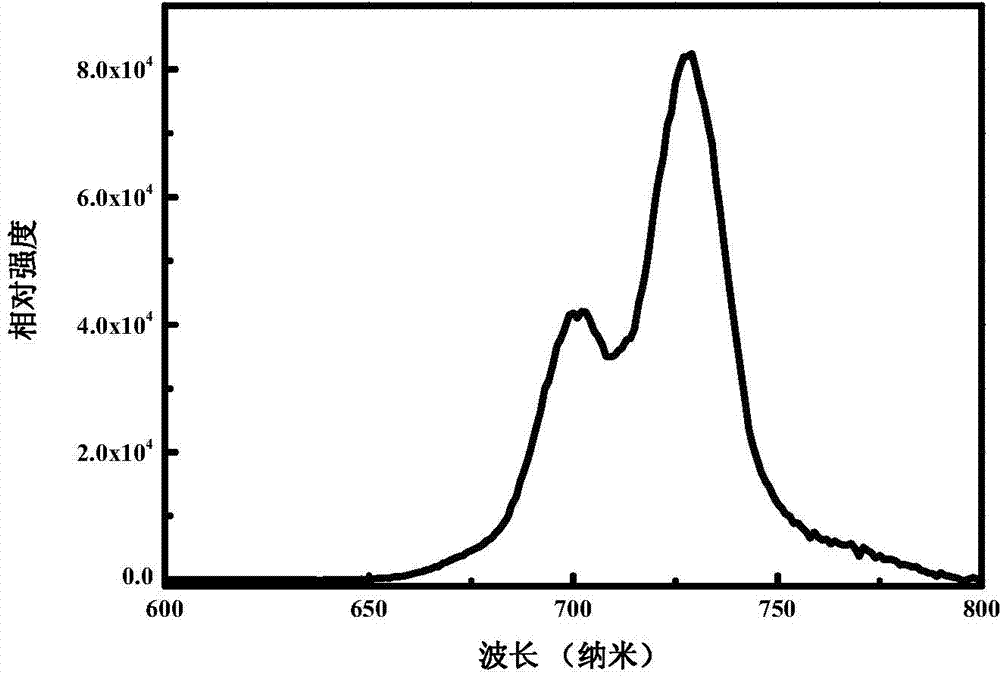
[0033] The fluorescence spectrum of the sample prepared in this embodiment is as follows: figure 1 As shown, fluorescence at 600nm-800nm is emitted under excitation at 336nm, and the luminescence peaks are at 731nm, 725nm, 717nm, 710nm, 704nm, 698nm, and 691nm. figure 2 The excitation spectra corresponding to all the emission peaks are shown in , and the excitation peaks corresponding to all the monitored emission peaks are 336nm and a broad peak at 450nm-550nm with a peak of 500nm. image 3 The long afterglow fluorescence spectrum of the sample prepared in this embodiment is shown for 1 minute after being irradiated by sunl...
Embodiment 2
[0036] According to the following composition: near-infrared long afterglow luminescent material LaAlO 3 :Mn x , Ge n , where the doping amount x of Mn ions is 0.3mol%, and the doping amount n of germanium ions is 1mol%; respectively weigh lanthanum oxide, aluminum oxide, manganese carbonate, and germanium oxide, and pre-calcine at 900°C after grinding and mixing Take it out after 1 hour, grind it again, and fire it at 1450°C for 4 hours.
[0037] The fluorescence spectrum and excitation spectrum of the sample prepared in this embodiment are as follows: Figure 7The display shows that under the excitation of 336nm, the fluorescence at 600nm-800nm is emitted, and the luminescence peaks are located at 731nm, 725nm, 717nm, 710nm, 704nm, 698nm, 691nm. The corresponding excitation peaks of all monitored emission peaks are 336nm and one 450nm-550nm, the peak is a broad peak at 500nm. Figure 8 The sample prepared in this embodiment was irradiated by sunlight for 10 minutes and...
Embodiment 3
[0039] According to the following composition: near-infrared long afterglow luminescent material LaAlO 3 :Mn x , Fe n , where the doping amount x of Mn ions is 0.5 mol%, and the doping amount n of iron ions is 5 mol%; respectively weigh lanthanum oxide, aluminum oxide, manganese carbonate, and iron oxide, and pre-calcine at 850°C after grinding and mixing Take it out after 12 hours, grind it again, and fire it at 1400°C for 5 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com