Preparation method of Cu-Ir alloy polyhedral nano cage
A technology of polyhedron and nanocage, which is applied in the field of preparation of Cu-Ir alloy nanocage particles, can solve the problems of high price and catalytic activity limitation, and achieve the effect of electrocatalytic activity of high oxygen generation reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: Preparation of elemental copper nanocrystals
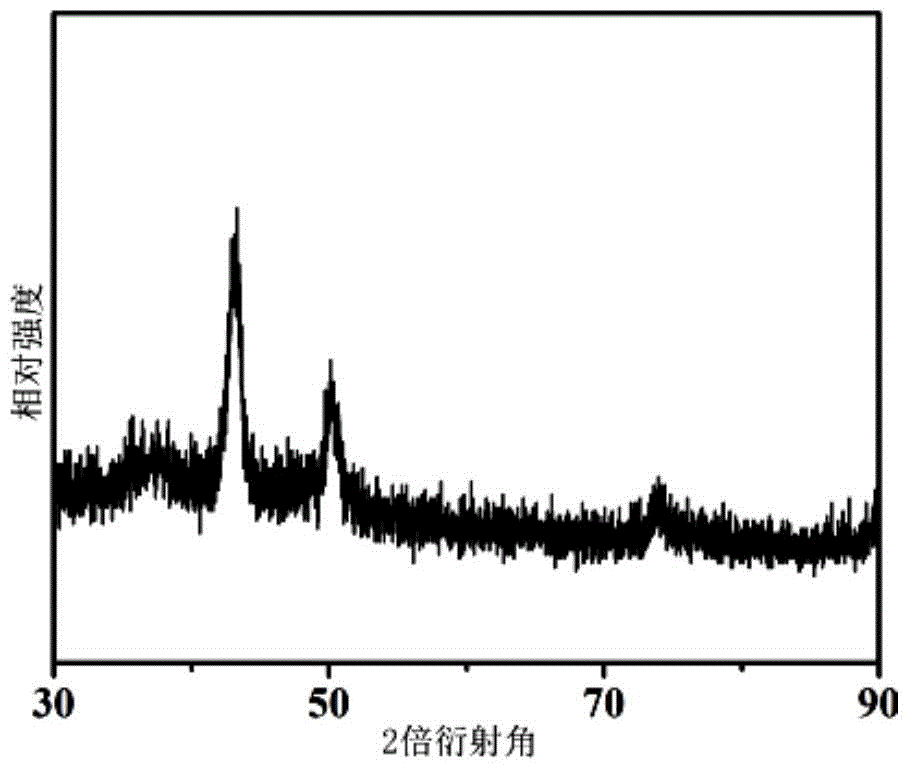
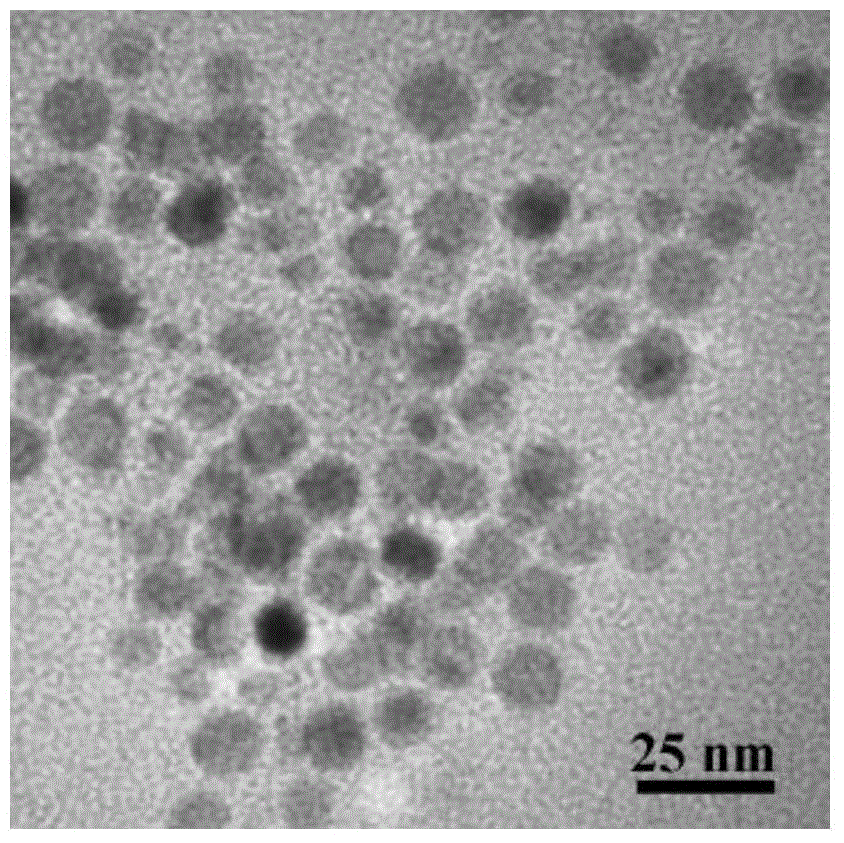
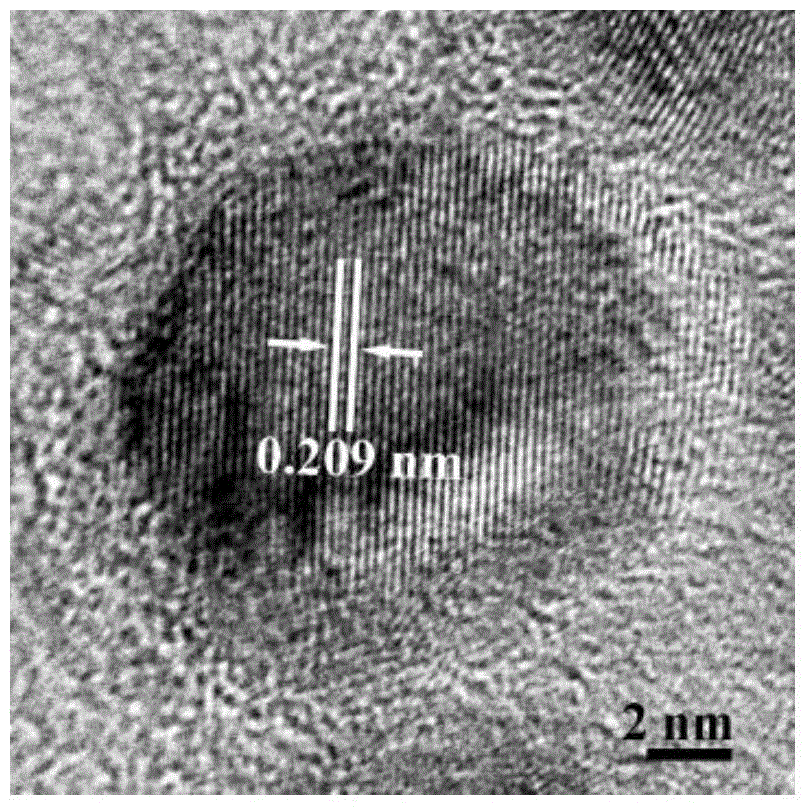
[0036] Put 0.2mmol copper acetylacetonate and 5mL oleylamine into a round-bottomed three-neck flask, and gradually raise the temperature to 270°C under magnetic stirring and nitrogen protection. During the heating process, the color of the solution changes from dark green to orange. When 270°C was reached, it turned into a wine-red cloudy solution, indicating the formation of copper nanocrystals, and was then held at 270°C for 15 minutes. figure 1 , figure 2 and image 3 They are the X-ray diffraction pattern, the transmission electron microscope image and the high-resolution transmission electron microscope image of the elemental Cu nanocrystals prepared in this example, respectively. It can be seen that the obtained Cu templates are nearly spherical single crystal nanoparticles with a particle diameter of about 14 nm.
Embodiment 2
[0037] Example 2: Hollow Porous Structure Cu 1.1 Preparation of Ir nanocages
[0038] First, prepare a solution of iridium chloride oleylamine with a concentration of 0.2mol / L. The specific operation process is as follows: mix 0.2mmol iridium chloride powder and 1mL oleylamine into a 50mL round-bottomed three-necked bottle, and raise the temperature to 120°C under the protection of nitrogen. °C for 30 minutes.
[0039] According to Example 1, copper acetylacetonate was reacted in oleylamine at 270°C for 15 minutes and then rapidly dropped to 250°C, and then 1 mL of iridium chloride oleylamine solution with a prepared concentration of 0.2mol / L was taken out with a syringe and quickly injected into In the round-bottomed three-neck flask of Example 1, keep 250 DEG C for 2 hours, at this moment, the mixed solution turns into a black turbid liquid, indicating that the hollow Cu 1.1 Formation of Ir nanocage structures. Figure 4 , Figure 5 , Figure 6 and Figure 7 Respective...
Embodiment 3
[0040] Example 3: Cu with core-shell structure 1.4 Ir and Cu 2 Preparation of Ir nanoparticles
[0041] By changing the molar ratio (3:1 or 3:2) of the precursor copper acetylacetonate and iridium chloride, the preparation of Cu core Cu-Ir alloy shell nanoparticles can be realized. When the molar ratio of precursor copper acetylacetonate and iridium chloride is 3:1, Cu with core-shell structure is obtained 2 Ir, Figure 11 and Figure 12 Its transmission electron micrographs and high-resolution transmission electron micrographs. It can be seen that the obtained Cu 2 The particle size of Ir nanoparticles is uniform, there is a layer of Cu-Ir alloy shell on the surface and there are obvious etched pits. When the molar ratio of the precursor copper acetylacetonate and iridium chloride is 3:2, Cu with core-shell structure is obtained 2 Ir, Figure 13 and Figure 14 Its transmission electron micrographs and high-resolution transmission electron micrographs. It can be seen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com