Selective hydrogenation method for C2 fraction
A selective hydrogenation and fractionation technology, applied in the direction of hydrocarbons, chemical instruments and methods, purification/separation of hydrocarbons, etc., can solve the problem of weak complexation, insufficient loading of active components, and complicated process And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
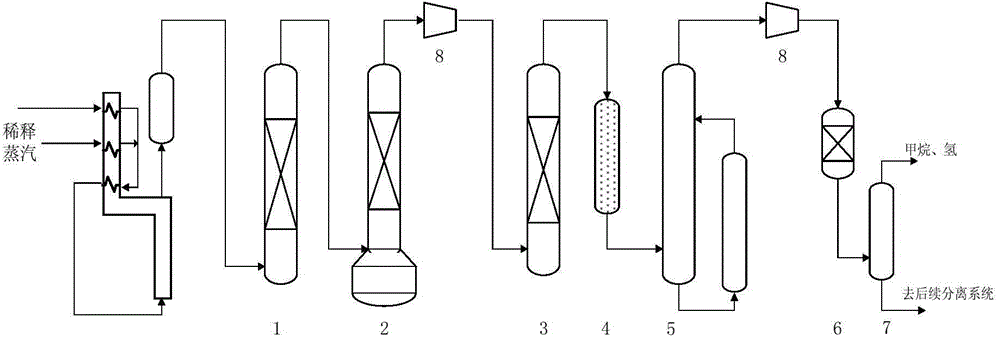
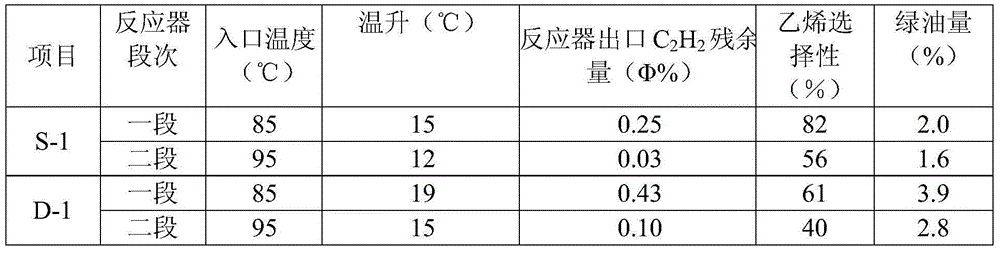
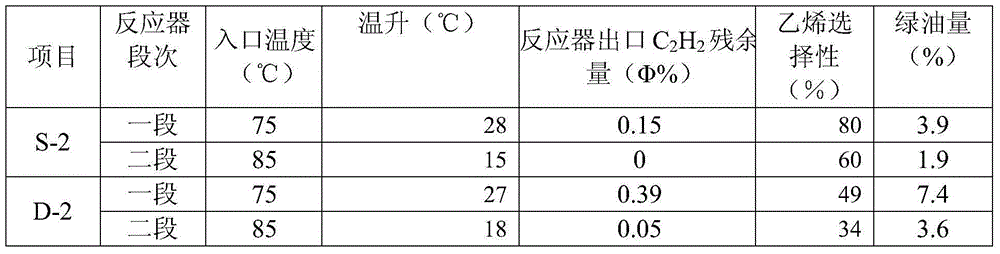
Image
Examples
Embodiment 1
[0053] Weigh Φ4.5mm, length is 4.5mm, specific surface area is 17m 2 / g, columnar α-Al with a pore volume of 0.22ml / g 2 o 3 Carrier 500g.
[0054] Dissolve 39.42g of 4,4-dihydroxy-2,2-bipyridine in 700mL ethanol solution, impregnate the above carrier in the above solution, and let it stand for 2 hours to fully load 4,4-dihydroxy-2,2-bipyridine After being placed on the alumina carrier, it was dried at 60°C for 10 hours to obtain hydroxy-bipyridine / Al 2 o 3 Prebody.
[0055] Weigh 0.18gPd(NO 3 ) 2 , 0.24gAgNO 3 , dissolved in 500mL of deionized water, added 10ml of nitric acid and stirred until completely dissolved, adjusted the pH value to 2.6, and prepared a mixed solution. The above-mentioned hydroxyl-bipyridine / Al 2 o 3 The precursor was added to the prepared solution, stirred for 10 minutes, left to stand for 2 hours, and the residue was poured out to obtain PdAg-hydroxyl-bipyridine / Al 2 o 3 Precursor (number of moles of hydroxyl-bipyridine: (Pd+Ag)=100). Afte...
Embodiment 2
[0074] Weigh Φ4.0×4.0mm, the specific surface area is 7m 2 / g, 500g of cylindrical carrier with a pore volume of 0.38ml / g, which contains α-Al 2 o 3 460g,TiO 2 40g.
[0075] Dissolve 104.89g of 4,4-dihydroxy-2,2-bipyridine in 800mL of ethanol solution, impregnate the above carrier in the above solution, let stand for 8 hours to fully load 4,4-dihydroxy-2,2-bipyridine After being placed on the alumina support, it was dried at 90°C for 8 hours to obtain hydroxy-bipyridine / Al 2 o 3 Prebody.
[0076] Weigh 0.49gPd(NO 3 ) 2 , 0.79gAgNO 3 , dissolved in 500mL deionized water, add 10ml nitric acid and stir until completely dissolved, adjust the pH value to 2.5, and prepare a mixed solution, the above-mentioned hydroxyl-bipyridine / Al 2 o 3 Add the precursor to the prepared solution, stir for 60 minutes, let it stand for 8 hours, pour out the residual liquid, and dry the remaining solid at 110°C for 4 hours to obtain PdAg-hydroxy-bipyridine / Al 2 o 3 Precursor (number of moles...
Embodiment 3
[0097] Weigh Φ3.7mm, the specific surface area is 20m 2 Al 2 o 3 It is a mixed crystal form of θ and α.
[0098] Dissolve 4.4g of 6,6'-dihydroxy-3,3'-bipyridine in 600mL ethanol solution, impregnate the above carrier in the above solution, and let 6,6'-dihydroxy-3,3'- After the bipyridine was completely loaded on the alumina carrier, it was dried at 120°C for 4 h to obtain the hydroxyl-bipyridine / Al 2 o 3 Prebody.
[0099] Weigh 0.61gPd(NO 3 ) 2 ,1.57gAgNO 3 , dissolved in 500mL of deionized water, added 10ml of nitric acid and stirred until completely dissolved, adjusted to pH 3.0, prepared into a mixed solution, the above-mentioned hydroxyl-bipyridine / Al 2 o 3 Add the precursor to the prepared solution, stir for 60 minutes, let it stand for 12 hours, pour out the residual liquid, and dry the remaining solid at 100°C for 8 hours to obtain PdAg-hydroxy-bipyridine / Al 2 o 3 Precursor (number of hydroxyl-bipyridine moles: (Pd+Ag)=2).
[0100] The precursors prepared a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com