Method for improving bioavailability of artemisinin-based drugs
An artemisinin and drug technology, applied in the field of improving the bioavailability of artemisinin drugs, can solve the problems of low bioavailability, unstable chemical properties, affecting drug efficacy and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Preparation of DHA bulk drug (dihydroartemisinin bulk drug) powder processed by SCF:
[0090] Supercritical fluid equipment of Tianjin Crystec Pharmaceutical Technology Co., Ltd. (composition: 200mL particle forming container, 50g / min-capacity CO 2 Pump). 2% DHA-ethanol solution (w / v), with CO 2 Pump into appropriate nozzles (coaxial nozzles), maintain a pressure of 85 bar (back pressure regulator control) and an operating temperature of 40°C, and finally collect the final powder in the forming chamber.
[0091] Characterization of SCF-treated DHA API powders:
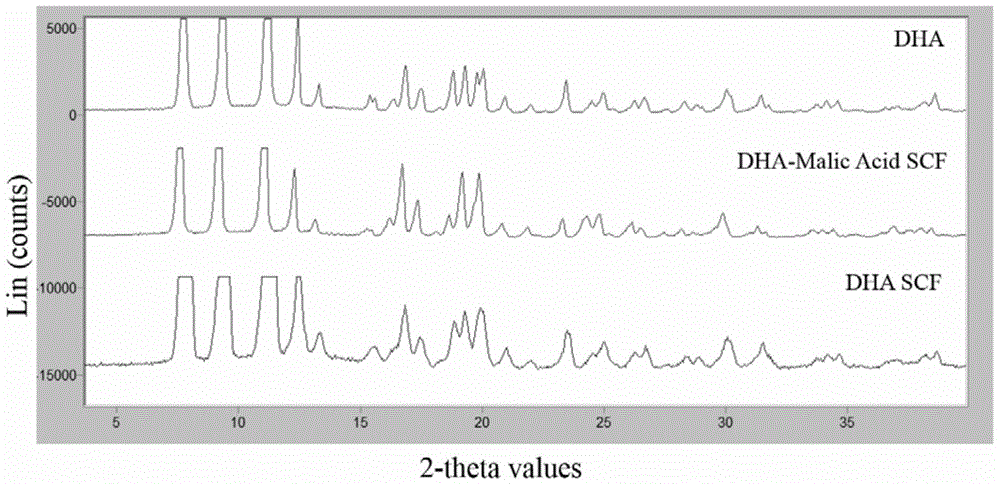
[0092] (1) Powder X-ray Diffraction (PXRD) characterization: Simultaneously compare and investigate DHA API, experimental instrument parameters: 40kV, 40mA; scanning speed 4θ / min. see results figure 1 . Both DHA API and SCF-treated DHA API have strong similar diffraction peaks at the same position, but the number of peaks and peak intensity have changed, and the crystallinity is strong.
[0093] (2) Scanni...
Embodiment 2
[0095] Preparation of DHA+malic acid powder treated by SCF: supercritical fluid equipment (composition: 200mL particle forming container, 50g / min-capacity CO 2 Pump). 12% (w / v) DHA and 8% (w / v) malic acid in dichloromethane-tetrahydrofuran (volume ratio 3:1) solution, with CO 2 Pump into appropriate nozzles, maintain a pressure of 85 bar and an operating temperature of 40°C, and finally collect the final powder in the forming chamber.
[0096] (1) Powder X-ray Diffraction (PXRD) characterization: Experimental instrument parameters: 40kV, 40mA; scanning speed 4θ / min. see results figure 1 , compared with the DHA raw material drug, there are stronger similar diffraction peaks at the same position, but the peak number and peak intensity are changed, and the crystallinity is stronger.
[0097] (2) Scanning electron microscope (SEM) characterization: see the results Figure 4 , all form good needle crystals, the average length of the "needle" crystal form is 82 μm, and the geome...
Embodiment 3
[0113] Preparation of DHA+gentisic acid co-crystallized powder processed by SCF: supercritical fluid equipment (composition: 200mL particle forming container, 50g / min-capacity CO 2 Pump). 5% DHA + 2% gentisic acid in dichloromethane, with CO 2 Pump into appropriate nozzles, maintain a pressure of 85 bar and an operating temperature of 36°C, and finally collect the final powder in the forming chamber. Scanning electron microscope (SEM) characterization results are shown in Figure 6 . The average crystal length is 71 μm, and the geometric equivalent diameter (particle size) is 10 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap