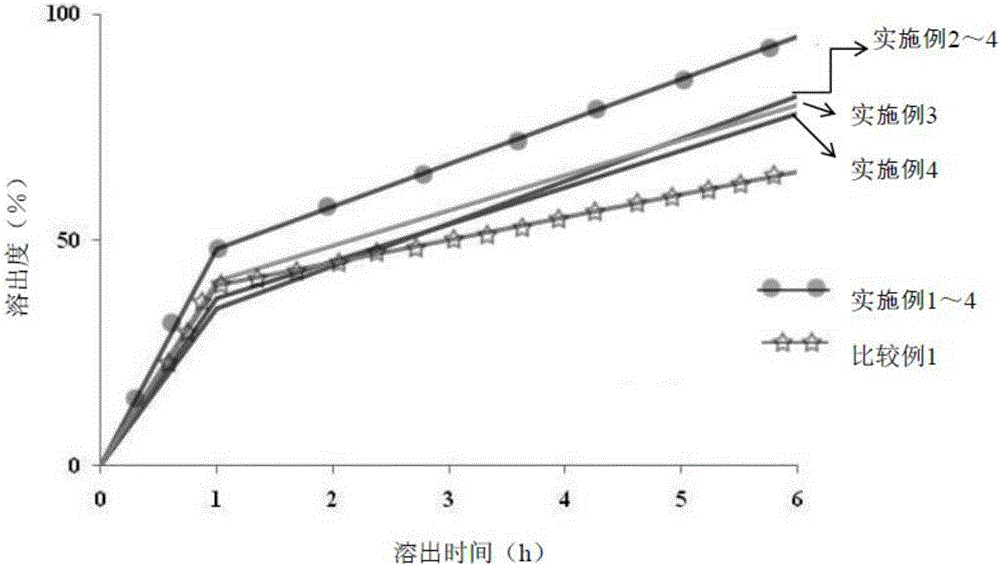
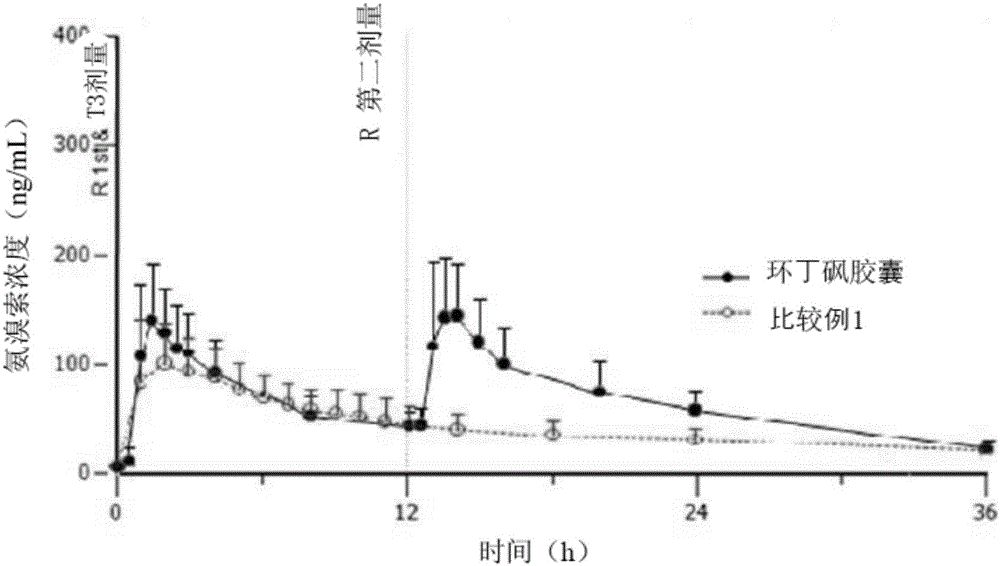
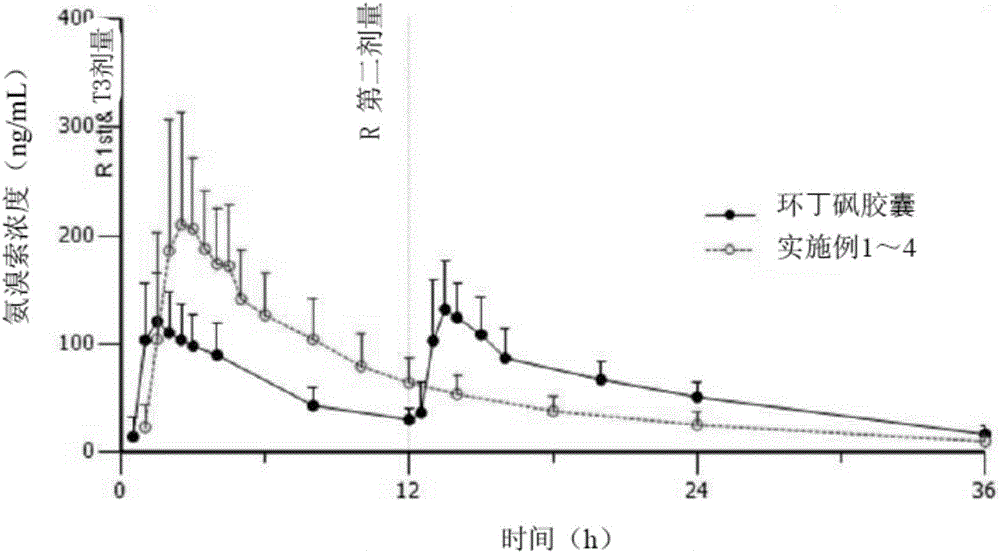
Sustained-release pharmaceutical composition containing acebrophylline and hydrophobic sustained-release agent
A kind of technology of bromoacetphylline and composition, applied in the field of sustained-release pharmaceutical composition, can solve the problems such as patient's pain, inability to achieve therapeutic effect, irregular administration time and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Preparation of Hydrophobic Sustained Release Formulations Comprising Ethylcellulose
[0060] The ingredients 1 to 4 of Table 1 were mixed and granulated, respectively. Components 5 to 7 in Table 1 were added to the granulated granules, mixed, and tabletted. After tableting, coating was performed with the coating agent No. 8 in Table 1.
[0061] Table 1
[0062]
Embodiment 2
[0063] Example 2: Preparation of Hydrophobic Sustained Release Formulations Comprising Waxes
[0064] The ingredients of 1 to 4 in Table 2 were mixed and granulated, respectively. Components 5 to 6 in Table 2 were added to the granulated granulated product, mixed, and tabletted. After tableting, coating was performed with the coating agent 7 in Table 2.
[0065] Table 2
[0066]
Embodiment 3
[0067] Example 3: Preparation of Hydrophobic Sustained Release Formulation Containing Monoglyceryl Behenate
[0068] The ingredients 1 to 4 of Table 3 were mixed and granulated, respectively. Components 5 to 6 in Table 3 were added to the granulated granulated product, mixed, and tabletted. After tableting, the coating agent 7 in Table 3 was used for coating.
[0069] table 3
[0070]
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap