Synthesis and Application of a Novel Iridium Metal Complex Phosphorescent Material
A technology of iridium metal complexes and metal complexes, applied in the direction of luminescent materials, indium organic compounds, platinum group organic compounds, etc., can solve the problem that red light organic phosphorescent materials cannot have high efficiency, saturated color purity and excellent life at the same time And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Synthesis of intermediate 2-(3-isoquinolyl)-6-methylbenzoxazole (A-1):
[0090]
[0091] Under the nitrogen protection system, put 25mmol of polyphthalamide (PPA) into the reaction system, raise the temperature to 100°C, add 25mmol of isoquinoline-3-carboxylic acid, 25mmol of 6-amino-m-cresol, At the same time, it was added to the above reaction solution, continued to heat up to 180°C, and reacted for 4 hours. After the reaction was completed, it was cooled, extracted with dichloromethane, and the solvent was evaporated by a rotary evaporator to obtain 2-(3-isoquinolinyl)- 6-methylbenzoxazole (A-1) 15.75mmol, yield 63%.
Embodiment 2
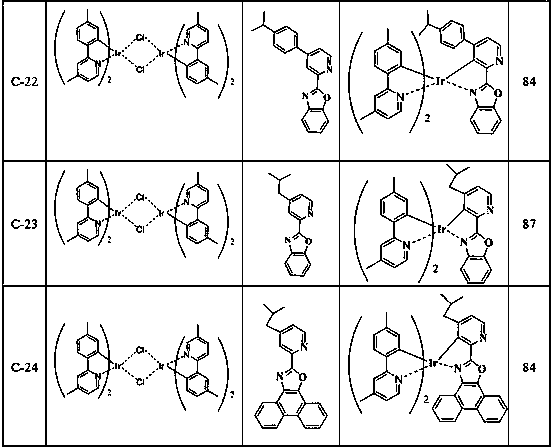
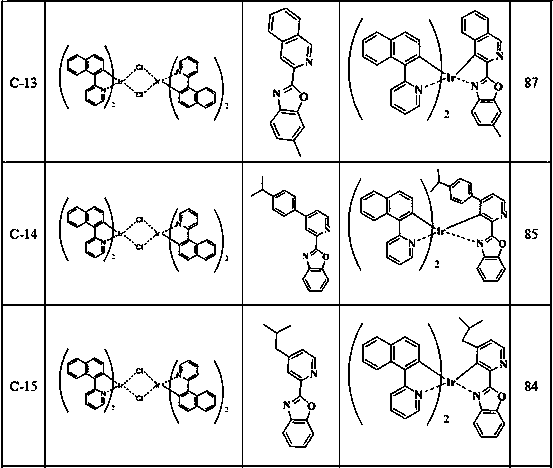
[0093] Intermediates A-2 and A-4 were synthesized according to the preparation method of intermediate A-1 above, and Table 1 is a summary of the reaction substances, generated substances and yields of Example 2 of the present invention.
[0094] Table 1 embodiment 2 reaction substance, generated substance and productive rate summary
[0095]
Embodiment 3
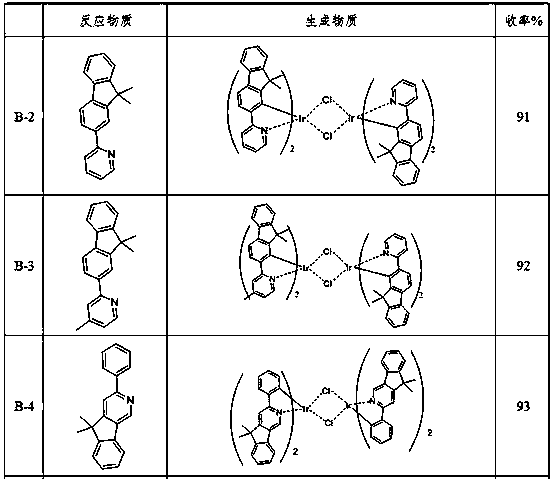
[0097] Synthesis of intermediate phenylpyridine metal iridium bridged ligand (B-1):
[0098]
[0099] Under a nitrogen protection system, weigh 22 mmol of 4-methyl-2,5-diphenylpyridine, iridium trichloride (IrCl 3 ·3H 2 O) Put 10mmol into the reaction system, add a mixed solution of 300ml ethylene glycol ethyl ether and 100ml pure water, reflux for 24 hours under nitrogen protection, then cool to room temperature, a precipitate precipitates, filter the precipitate with suction, rinse with water, and dry. Using dichloromethane as a detergent, silica gel column chromatography, concentrated and precipitated solids to obtain a novel iridium metal complex metal iridium bridging ligand (B-1) of oxazoles (9.2 mmol, 92%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com