d-a type small molecule compound and its preparation method and application
A small molecular compound, D-A technology, applied in the field of solar cells, achieves the effect of strong transfer ability, stable positive ions, and enhanced electron donating ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The present invention also provides a preparation method for the D-A type small molecule compound described in the above technical scheme, comprising the following steps:
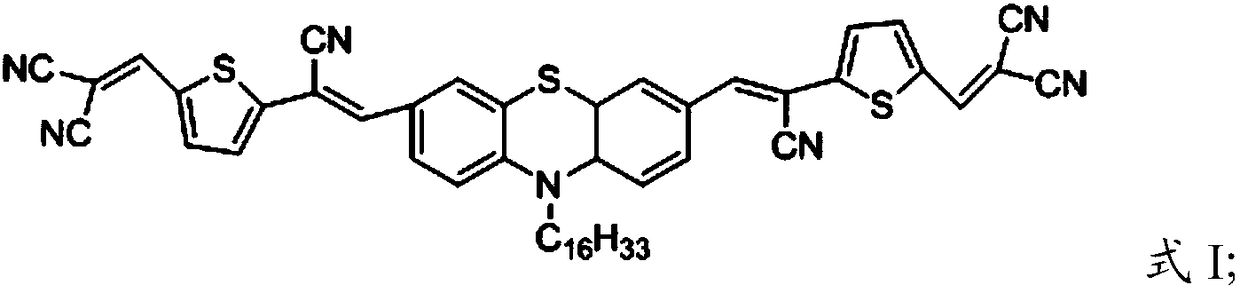
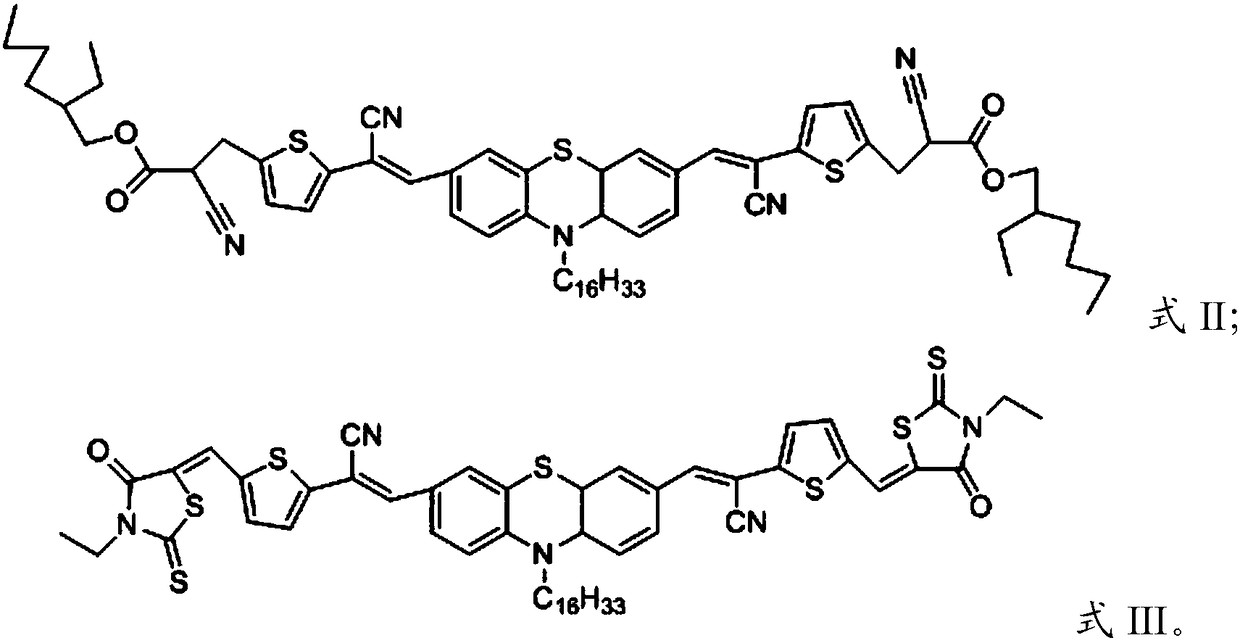
[0029] Under a protective gas atmosphere, the compound having the structure shown in formula IV, the electron-withdrawing monomer and the catalyst are reacted in an organic solvent system to obtain the D-A small molecule compound with the structure shown in formula I, formula II or formula III;
[0030]
[0031] The electron-withdrawing monomer is malononitrile, 2-ethylhexyl cyanoacetate or 3-ethylrhodanine.
[0032] In the present invention, the compound having the structure shown in formula IV, the electron-withdrawing monomer and the catalyst are reacted in an organic solvent system under a protective gas atmosphere to obtain the D-A type small molecule compound described in the above technical scheme. In the present invention, when the electron-withdrawing monomer is malononitrile, the prepare...
Embodiment 1
[0096] Under argon protection, 0.2 g (0.277 mmol) of a compound represented by the formula IV, 5 mL of CH 2 Cl 2 and 54mg of malononitrile (0.831mmol); 15mg of L-alanine was dissolved in 4.5mL of absolute ethanol, and the resulting L-alanine solution was added dropwise to the flask, and the reaction was stirred at 40°C overnight;
[0097] Add 30mL of water to the resulting reaction solution and add 50mLCH 2 Cl 2 For extraction, the obtained organic layer was washed three times with 0.36 g / mL brine successively, filtered, and the solvent was distilled off under reduced pressure at a vacuum degree of 0.1 MPa and a distillation temperature of 35° C. to obtain a crude product;
[0098] The crude product that obtains is carried out column chromatography purification, and column chromatography adopts silica gel column, and the eluent of column chromatography is the CH that volume ratio is 2:1 2 Cl 2 and n-hexane to obtain 0.098 g of dark red solid.
[0099] The yield of the pr...
Embodiment 2
[0104] Under argon protection, 0.2 g (0.277 mmol) of a compound having a structure shown in Formula IV, 28 mL of CH 2 Cl 2 , 1.35mL (6.73mmol) 2-ethylhexyl cyanoacetate and catalytic amount of triethylamine, the resulting mixed solution was stirred and reacted at room temperature for 12h;
[0105] The obtained reaction solution was distilled off under reduced pressure to remove the solvent under the conditions of a vacuum degree of 0.1 MPa and a distillation temperature of 35° C. to obtain a crude product;
[0106] The obtained crude product is purified by column chromatography, the column chromatography adopts a silica gel column, and the eluent of the column chromatography is CH with a volume ratio of 2:1. 2 Cl 2 and n-hexane to obtain 0.15 g of dark red solid.
[0107] The yield rate of the product calculated by the present invention is 50%.
[0108] The present invention carries out proton nuclear magnetic resonance spectrum measurement with the deep red solid that obt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com