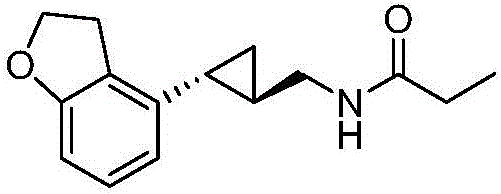
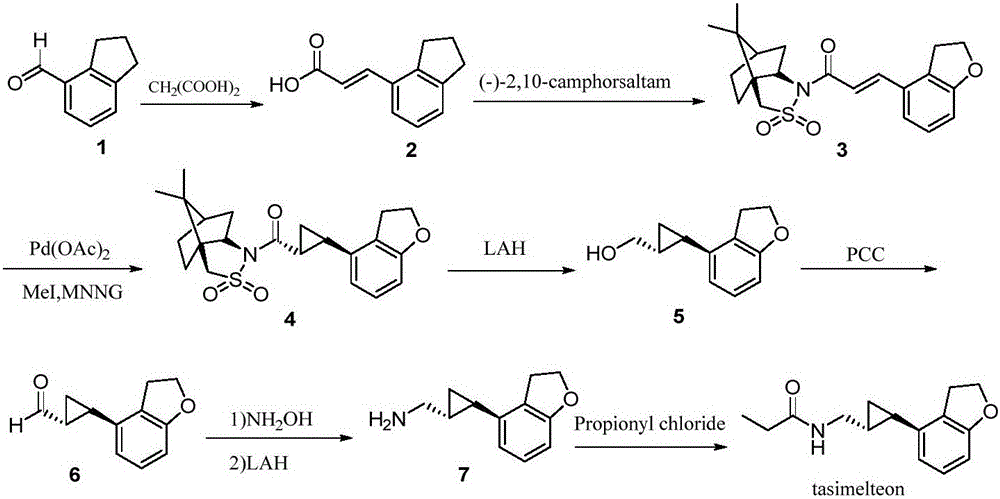
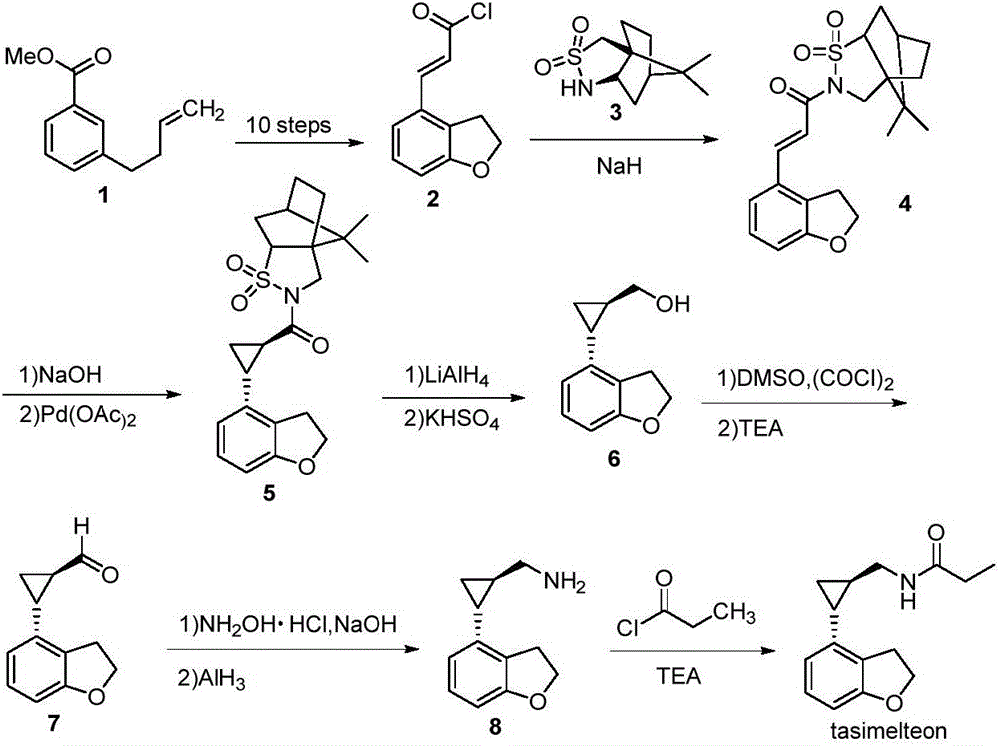
Synthesis method of tasimelteon
A synthetic method, the technology of tasimelteon, is applied in the field of new synthesis of the anti-insomnia drug tasimelteon, which can solve the problems of difficult large-scale industrial production, unstable quality of finished products, harsh reaction conditions, etc., and achieve mild reaction conditions, The effect of good product quality and simple route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: the preparation of compound (II)
[0054] Take a 100mL single-necked flask, add 4-vinyl-2,3-dihydrobenzofuran (5.0g, 34.2mmol), acetonitrile (25mL), 30% hydrogen peroxide (20.8mL), sodium bicarbonate (11.5g, 136.8 mL), stirred at room temperature for 10 h, and TLC monitored that the reaction was complete. Add saturated sodium bisulfite solution dropwise under ice-bath conditions to quench, then extract with ethyl acetate (100mL), wash the organic phase with water (50mL), saturated brine (30mL), concentrate under reduced pressure, column chromatography (washing The removal agent was ethyl acetate:petroleum ether=10:1, v:v) Purification gave 5.0 g of yellow liquid compound (II) with a yield of 91%.
[0055] 1 H NMR (500MHz, CDCl 3 ):δ=2.84(dd,J 1 =2.6Hz,J 2 =5.6Hz,1H),3.14(dd,J 1 =4.2Hz,J 2 =5.6Hz,1H),3.27(t,J=8.7Hz,2H),3.84-3.86(m,1H),4.60(t,J=8.8Hz,2H),6.71-6.76(m,2H),7.12 (t,J=7.9Hz,1H).
Embodiment 2
[0056] Embodiment 2: the preparation of compound (II)
[0057] Take a 50mL single-necked flask, add 4-vinyl-2,3-dihydrobenzofuran (3.0g, 20.5mmol), dichloromethane (15mL), potassium carbonate (15.6g, 113.0mmol), add it while stirring at room temperature 60% m-chloroperoxybenzoic acid (10.6 g, 61.5 mmol) was solid, stirred for 10 h, and the reaction was complete by TLC monitoring. Add dropwise saturated sodium bisulfite solution to quench, then extract with ethyl acetate (50mL), wash the organic phase with water (30mL), saturated brine (20mL), concentrate under reduced pressure, column chromatography (eluent: Ethyl acetate:petroleum ether=10:1, v:v) After purification, 2.7g of yellow liquid compound (II) was obtained with a yield of 80%.
[0058] 1 H NMR (500MHz, CDCl 3 ):δ=2.84(dd,J 1 =2.6Hz,J 2 =5.6Hz,1H),3.14(dd,J 1 =4.2Hz,J 2 =5.6Hz,1H),3.27(t,J=8.7Hz,2H),3.84-3.86(m,1H),4.60(t,J=8.8Hz,2H),6.71-6.76(m,2H),7.12 (t,J=7.9Hz,1H).
Embodiment 3
[0059] Embodiment 3: the preparation of compound (III)
[0060] Take a 50mL three-necked flask, completely dissolve compound (II) (2.0g, 12.3mmol) in tetrahydrofuran (15mL), add diethyl cyanomethyl phosphate (2.2g, 12.4mmol), sodium methoxide (5.3g, 98.1mmol) , vacuumed and protected by nitrogen, refluxed at 80° C., reacted for 8 hours, and the reaction was complete as monitored by TLC. The reaction solution was cooled to room temperature, water (10 mL) was added, and then extracted with ethyl acetate (50 mL), and the extracted organic phase was washed with water (30 mL) and saturated brine (30 mL). The collected organic phase was concentrated under reduced pressure to obtain a brown viscous substance, which was purified by column chromatography (eluent: ethyl acetate:petroleum ether=8:1, v:v) to obtain 2.1 g of a white solid with a yield of 92%.
[0061] FT-IR(KBr)υ:773,984,1237,1439,1457,1478,1592,1612,2238,2898,2971cm -1 ; 1 HNMR (500MHz, CDCl 3 ):δ=1.40-1.48(m,1H),1.54...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap