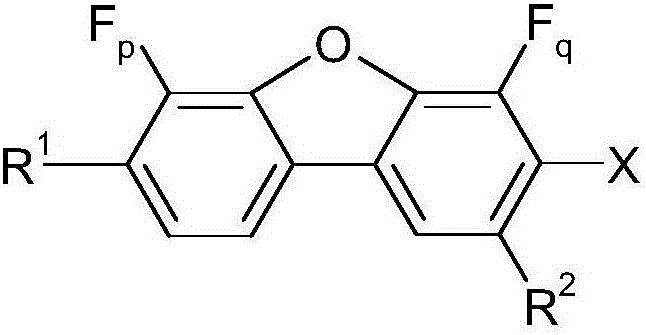
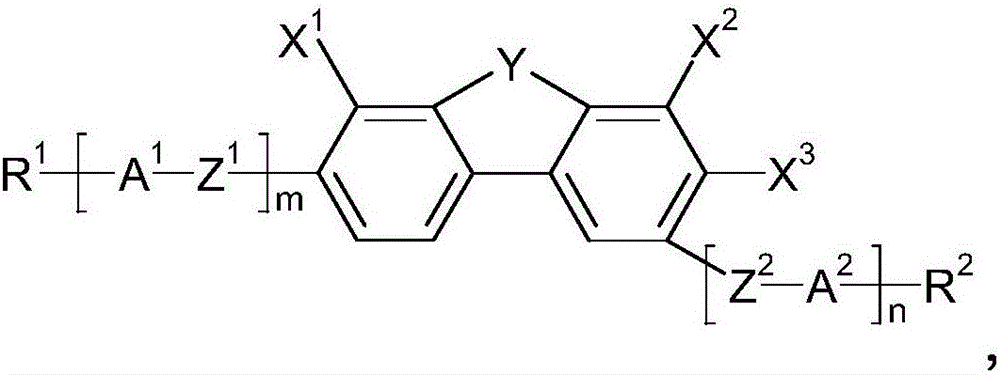
Fluorinated dibenzofuran and dibenzothiophene derivative
A compound and phenylene technology, applied in the field of electro-optic display elements, can solve problems such as unfinished development in the field of liquid crystal materials, and achieve the effect of good width
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0111] The present invention is explained in more detail below with reference to Examples, but it is not intended to be limited thereto. Those skilled in the art will be able to glean from the examples operational details not given in detail in the general description, generalize them based on general expertise and apply them to specific problems.
[0112] In addition to the usual and well-known abbreviations, the following abbreviations are used:
[0113] C: liquid crystal phase; N: nematic phase; Sm: smectic phase; I: isotropic phase. The numbers between these symbols show the transition temperature of the substances involved.
[0114] Temperature data are in °C unless otherwise stated.
[0115] By generally known methods, as described inter alia in the handbook "Merck Liquid Crystals- - Methods in Physical Properties of Liquid Crystals - Description of the Measurement Methods", 1998, Merck KGaA, Darmstadt for the determination of physical, physicochemical or electro-opt...
Embodiment 1
[0128] Example 1: 4,6-Difluoro-3-trifluoromethoxy-7-pentyloxydibenzofuran
[0129] 1.1: 1,2-Difluoro-3-pentyloxybenzene
[0130]
[0131] 19.0 g (146 mmol) of 2,3-difluorophenol and 20.1 ml (163 mmol) of 1-bromopentane were dissolved in ethyl methyl ketone, 22.4 g of potassium carbonate were added, and the mixture was heated at boiling overnight . A solid then separates out and the solvent is removed. The amyl 2,3-difluorophenyl ether obtained was used without further purification in the subsequent step.
[0132] 1.2: 2,3-Difluoro-4-pentyloxyphenylborane acid
[0133]
[0134] 108 ml of a 15% solution of n-butyllithium in n-hexane (172 mmol) were added to a solution of 31.2 g of the crude product (10) from step 1 in 225 ml of THF at -70°C. After 1 hour at this temperature, 20 ml (176 mmol) of trimethylborate dissolved in 25 ml of THF were added. After an additional hour, the batch was warmed to 0° C., water was added, and the mixture was adjusted to pH 1 using 2...
Embodiment 25
[0165] Example 25 : Butoxy-4,6-difluoro-7-trifluoromethoxydibenzothiophene
[0166] 25.1 :3,2',3'-Trifluoro-4'-butyloxy-4-trifluoromethoxybiphenyl-2-ol
[0167]
[0168] Phenol 22 was prepared analogously to compound 14 by Suzuki coupling of compound 13 and commercially available 4-butoxy-2,3-difluorophenylborane acid.
[0169] 25.2 :3,2',3'-Trifluoro-4'-butyloxy-4-trifluoromethoxybiphenyl-2-yl trifluoromethanesulfonate
[0170]
[0171] Add triethylamine (5.0ml) and DMAP (60mg) dropwise to 3,2',3'-trifluoro-4'-butyloxy-4-trifluoromethoxybiphenyl-2-ol (9.7g) in a solution in dichloromethane (70ml). Trifluoromethanesulfonic anhydride (5.0 ml) was then added dropwise at 5°C, and the reaction mixture was stirred at room temperature for 20 hours. The mixture was filtered through silica gel, washed with dichloromethane, dried (Na 2 SO 4 ) and evaporated under vacuum. 3,2',3'-Trifluoro-4'-butyloxy-4-trifluoromethoxybiphenyl-2-yl triflate 9 was isolated as a residue a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com