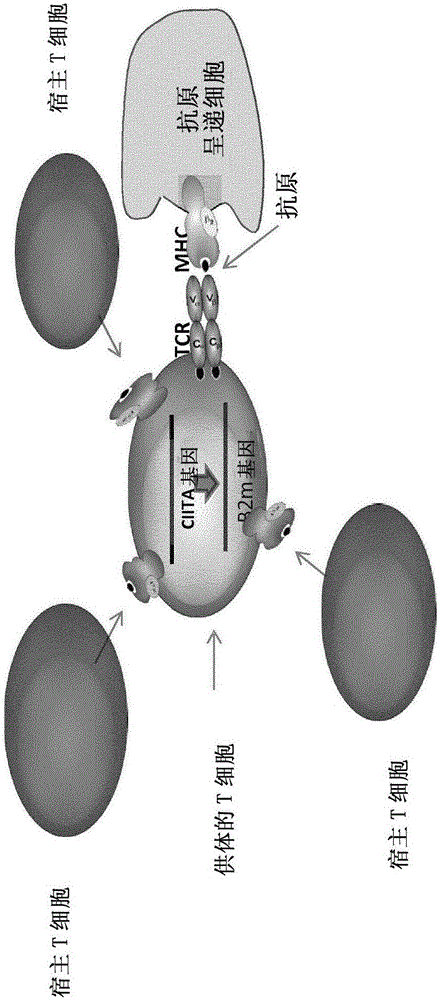
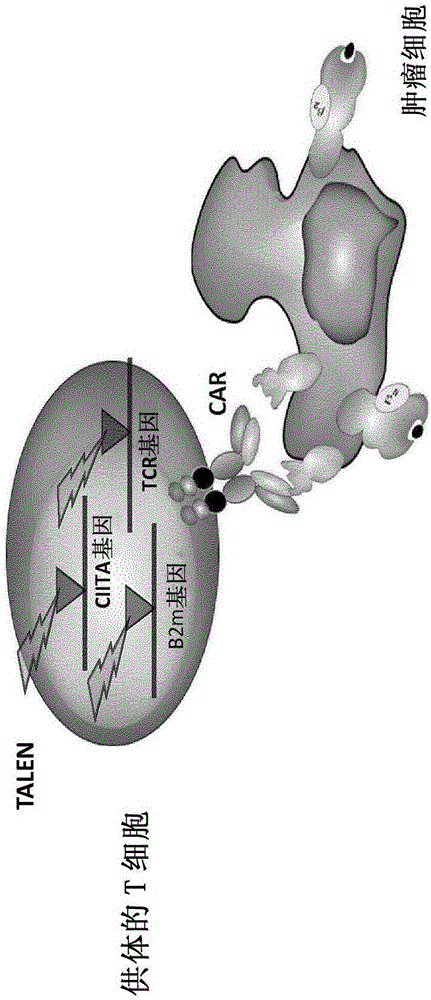
Method for generating T-cells compatible for allogenic transplantation
A cell and lymphocyte technology, applied in biochemical equipment and methods, animal cells, vertebrate cells, etc., can solve the problems of invisible host CTL, damage to MHCI, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
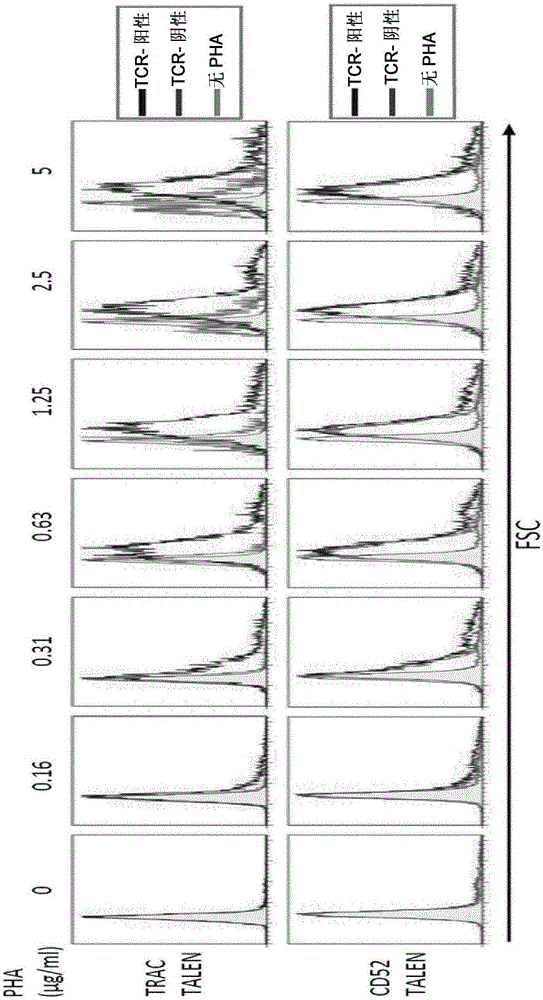
[0188] As a preferred embodiment of the present invention, for example, by electroporation, the nucleic acid molecule encoding the endonuclease of the present invention is transfected in the form of mRNA to obtain transient expression and avoid chromosomal integration of foreign DNA. The present inventors have identified different optimal conditions for mRNA electroporation in T cells presented in Table 1. The present inventors have used CytoPulse technology, which allows the delivery of substances into living cells by transiently permeating living cells using pulsed electric fields (US patents US6010613 and WO 2004 / 083379). Pulse duration, intensity, and interval between pulses can be modified to achieve optimal conditions for high transfection efficiency with minimal mortality. Essentially, the first high electric field pulse allows pore formation, while subsequent low electric field pulses allow the migration of the polynucleotide into the cell. In one aspect of the invent...
Embodiment
[0264] TALE nuclease that cleaves human CIITA
[0265] The mRNA encoding the TALE nuclease targeting the exons of the human CIITA gene was ordered from Cellectis Bioresearch (8, rue de la Croix Jarry, 75013 PARIS). Table 3 below indicates the target sequences cleaved by each of two independent entities (termed half-TALE nucleases), each containing a target sequence engineered to bind and cleaved by two separated by a 15bp spacer. Repeated sequences between target sequences consisting of open 17bp long sequences (called half-targets). Because exon 2 and exon 3 are shared by all transcript variants of CIITA, two TALEN pairs were designed for exon 2 and exon 3. The human genome has been predicted without significant dislocation targeting using TALE nucleases targeting these sequences.
[0266]
[0267]
[0268] Table 3: Description of CIITA TALE nucleases and their associated target sequences
[0269] TALE nuclease that cleaves human β2m
[0270] The mRNA encoding t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com