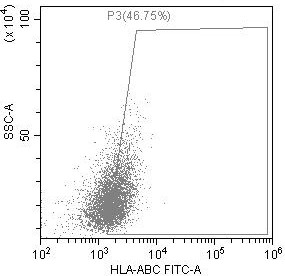
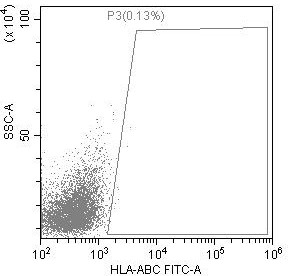
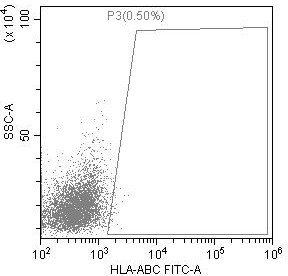
Preparation method of low-immunogenicity iPSC cell, low-immunogenicity iPSC cell and composition
A technology of immunogenicity and composition, applied in the field of cells, can solve the problems of difficult standardization, high-throughput production, cumbersome steps, etc., and achieve the effects of excellent differentiation effect, high positive expression rate, and time reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Construction of the vector
[0069] 1) Selection of CRISPR gene editing vector: The plasmid / vector that expresses Cas9 and sgRNA at the same time is selected from PX458 obtained from Addgene;
[0070] 2) Insertion of the target sgRNA into PX458: The sequence between the two BbsI sites (245 and 267) was replaced by the corresponding sgRNA sequence by conventional molecular cloning methods, that is, the corresponding target sgRNA vector was obtained.
[0071] A total of 4 sgRNA plasmids were constructed in the experiment, and the tables corresponding to their numbers and sgRNA sequences are shown in Table 1:
[0072] Table 1 4 sgRNAs
[0073] name / number use for site sequence B2M-sgRNA CRISPR vector B2M SEQ ID NO: 2 CIITA-sgRNA CRISPR vector CIITA SEQ ID NO: 8 AAVS1-sgRNA CRISPR vector AAVS1 SEQ ID NO: 21 AAVS1-CD47 sequence Homologous recombination template vector AAVS1 (insert CD47) SEQ ID NO: 20
[0074]...
Embodiment 2
[0076] Example 2 Electrotransfection
[0077] 2.1 iPSCs were cultured to 90% confluency using standard methods, iPSCs were cultured to 90% confluence using standard methods, cells were digested and counted.
[0078] 2.2 After counting the cells, remove 1×10 6 -1×10 7 cells (in this example, 1 × 10 6 ), centrifuge at low speed, take out the centrifuge tube to the biological safety cabinet, unscrew the cap of the tube, and discard the supernatant. By definition, low-speed centrifugation is a centrifugation with a rotational speed of 8,000 r / min or less and a relative centrifugal force of 10,000 × g or less.
[0079] 2.3 Absorb 0.5ug-5ug (preferably 0.5ug-2ug, in this example, 1ug) of the above four plasmids respectively, and add them into the electroporation buffer. The four plasmids absorb 0.5ug-5ug respectively, and can be designed as 0.5ug, 0.6ug, 0.7ug, 0.8ug, 0.9ug, 1ug, 1.1ug, 1.2ug, 1.3ug, 1.4ug, 1.5ug, 1.6ug, 1.7 ug, 1.8ug, 1.9ug, 2ug, 2.1ug, 2.2ug, 2.3ug, 2.4ug, 2....
Embodiment 3
[0081] Example 3 Electric transfer to pick clones
[0082] 3.1 Change 2ml Stemflex medium every day, after two days of culture, digest, count, and inoculate 5000 cells in a 10cm dish (Matrigel was pre-coated, and 10ml Stemflex+Rocki medium was added), Rocki in Stemflex+Rocki medium The concentration is 10 μM.
[0083] 3.2 After about 7 days of culture, single clones were picked in the laminar flow workbench. A total of 48 clones were picked and inoculated in two 24-well plates (Matrigel was pre-laid, and 500ul Stemflex+Rocki medium was added to each well). Continue to culture for about 7 days to verify positive clones.
[0084] 3.3 Identification of triple edited clones.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com