Preparation method of high-purity 12-methyl tridecaldehyde
A methyl tridecaldehyde and high-purity technology is applied in the field of preparation of high-purity 12-methyl tridecaldehyde, can solve the problems of long reaction steps, heavy metal pollution and high risk, achieves low cost, mild reaction conditions, The effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
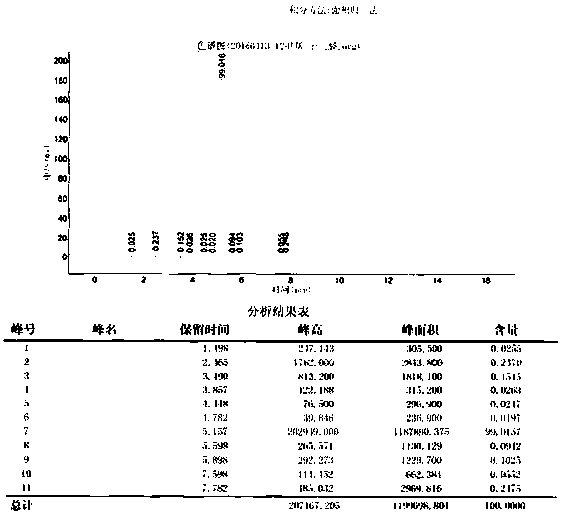
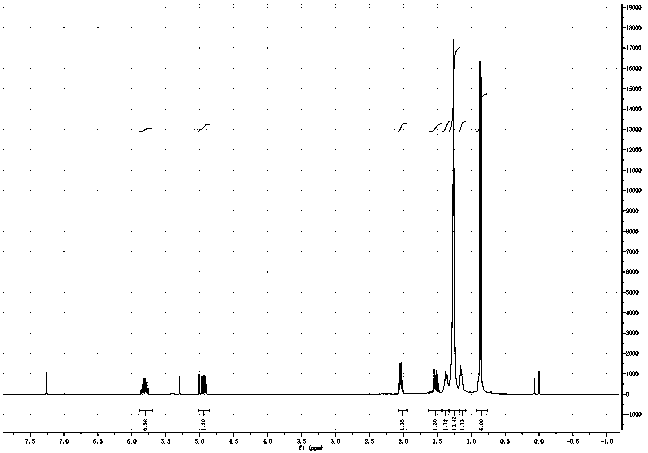
Image
Examples
preparation example Construction
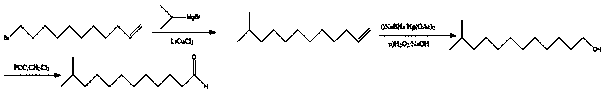
[0069] The synthetic route of the preparation method of described high-purity 12-methyl tridecaldehyde is as follows:
[0070] The preparation method of described high-purity 12-methyl tridecanal comprises the following steps:
[0071] A preparation method of high-purity 12-methyl tridecaldehyde, which uses 10-undecylenic acid as a raw material, undergoes four steps of amidation reaction, Grignard reaction, hydrogenation reduction and alkenyl ozonation, and obtains the final product , the specific operation is as follows:
[0072] Step one amidation reaction
[0073] At room temperature, add dichloromethane and 10-undecylenic acid to the reaction kettle, add thionyl chloride dropwise under stirring, and react at 10-40°C to detect the completion of the reaction, and then concentrate to obtain A; 10-undecylenic acid The mass volume ratio of enoic acid and dichloromethane is 1g:16~25ml; the molar ratio of 10-undecylenic acid and thionyl chloride is 1:2~4;
[0074]
[0075] ...
Embodiment 1
[0091] Step one amidation reaction
[0092] At room temperature, add 800ml of dichloromethane and 50g of 10-undecylenic acid into the reaction kettle, add 64.6g of thionyl chloride dropwise under stirring, and raise the temperature to 40°C to react after the dropwise completion, and then concentrate to obtain A;
[0093] At room temperature, add 291ml of dichloromethane and 29.1g of N,O-dimethylhydroxylamine hydrochloride to the reaction kettle, cool down to 0°C, add the dichloromethane solution of molecule A dropwise, and rise to 40°C to react after dropping;
[0094] GC detects the reaction, after the reaction is completed, add 150ml of dilute hydrochloric acid with a mass fraction of 20% to the reaction kettle dropwise, fully stir for 20-60min, separate the liquids, extract the aqueous phase with 270ml of dichloromethane in batches for three times, combine the dichloromethane phases, and dry. Concentrated to obtain 63.3gB, gas chromatography mass spectrometry purity 95.0%, ...
Embodiment 2
[0104] Step one amidation reaction
[0105] At room temperature, add 1250ml of dichloromethane and 50g of 10-undecylenic acid into the reaction kettle, add dropwise 129.1g of thionyl chloride under stirring, and react at 10°C to detect the completion of the reaction, and then concentrate to obtain A;
[0106] At room temperature, add 344ml of dichloromethane and 34.4g of N,O-dimethylhydroxylamine hydrochloride to the reaction kettle at one time, cool down to 5°C, add the dichloromethane solution of molecule A dropwise, and rise to 10°C for reaction ;
[0107] GC detects the reaction, after the reaction is completed, add 400ml of 5wt% dilute hydrochloric acid dropwise to the reaction kettle, fully stir for 20-60min, separate the liquids, extract the water phase with 300ml dichloromethane in batches for three times, combine the dichloromethane phases, dry, concentrate, Obtained 62.0g B, gas phase mass spectrometry purity 96.3%, yield 96.8%;
[0108] Step 2 Grignard reaction
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com