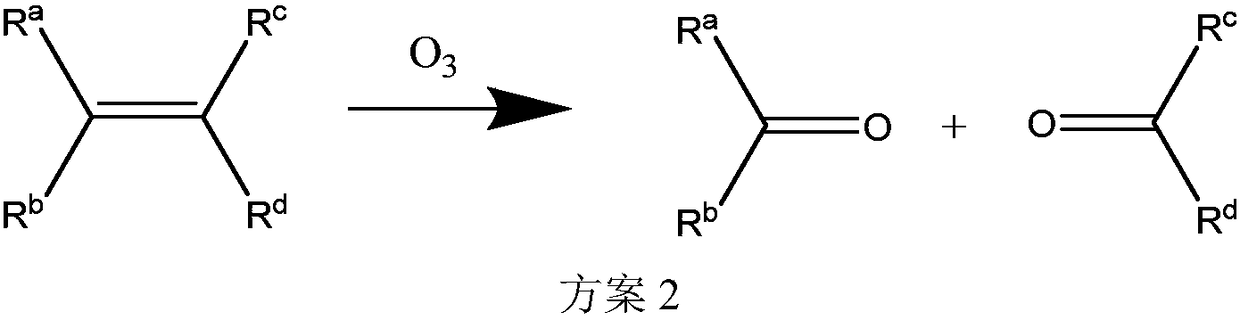
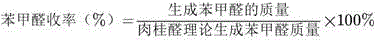Patents
Literature
128results about "Preparation by ozonolysis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method of natural benzaldehyde
InactiveCN102826978AGood choiceHigh purityPreparation by ozonolysisMetal/metal-oxides/metal-hydroxide catalystsBenzaldehydeDistillation
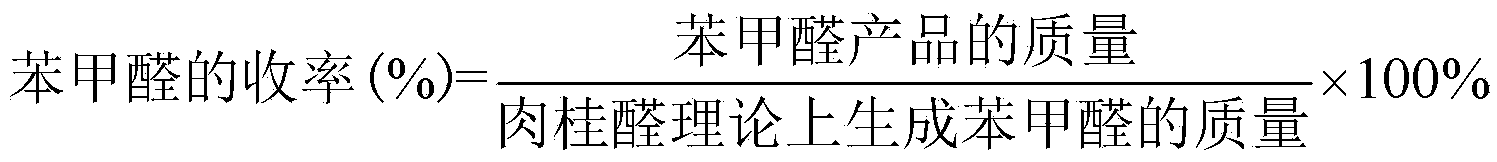
The invention discloses a preparation method of natural benzaldehyde. The method comprises the following steps of: getting cinnamyl aldehyde or cinnamon oil as a raw material; adding one or more of multi-phase catalysts of 0.5% to 10% of MnO2, TiO2, Al2O3, SnO2, Fe2O3, MgO, CuO, CeO2, ZrO2, Bi2O3, Y2O3 or active carbon; pouring 0.05 to 0.5g of ozone in a bubbling reactor at -5 to 20 DEG C based on 1g of cinnamyl aldehyde per hour; carrying out an ozonization reaction for 0.5 to 10 hours to obtain an ozonide intermediate; dropping the ozonide intermediate into the thiourea aqueous solution to be reduced while agitating at a constant low temperature, so as to obtain an oil-water mixture; separating the oil from the water to obtain a rough benzaldehyde product; and finally operating a molecular distillation device to obtain the benzaldehyde with relatively high purity. The preparation method has the advantages of simple technology, green reaction, being capable of remaining the natural property of the benzaldehyde, high selectivity, and high yield of the benzaldehyde.
Owner:GUANGXI UNIV
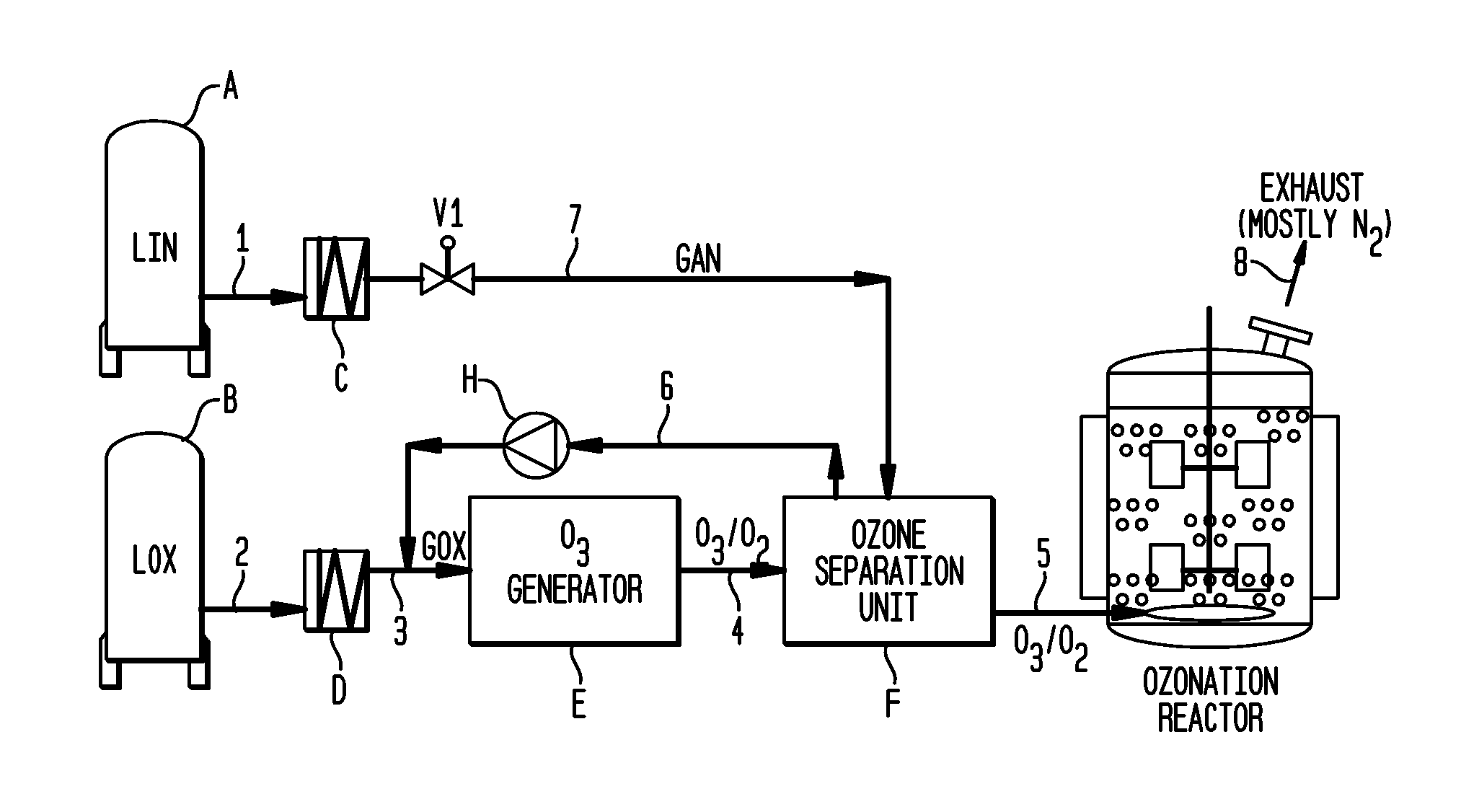
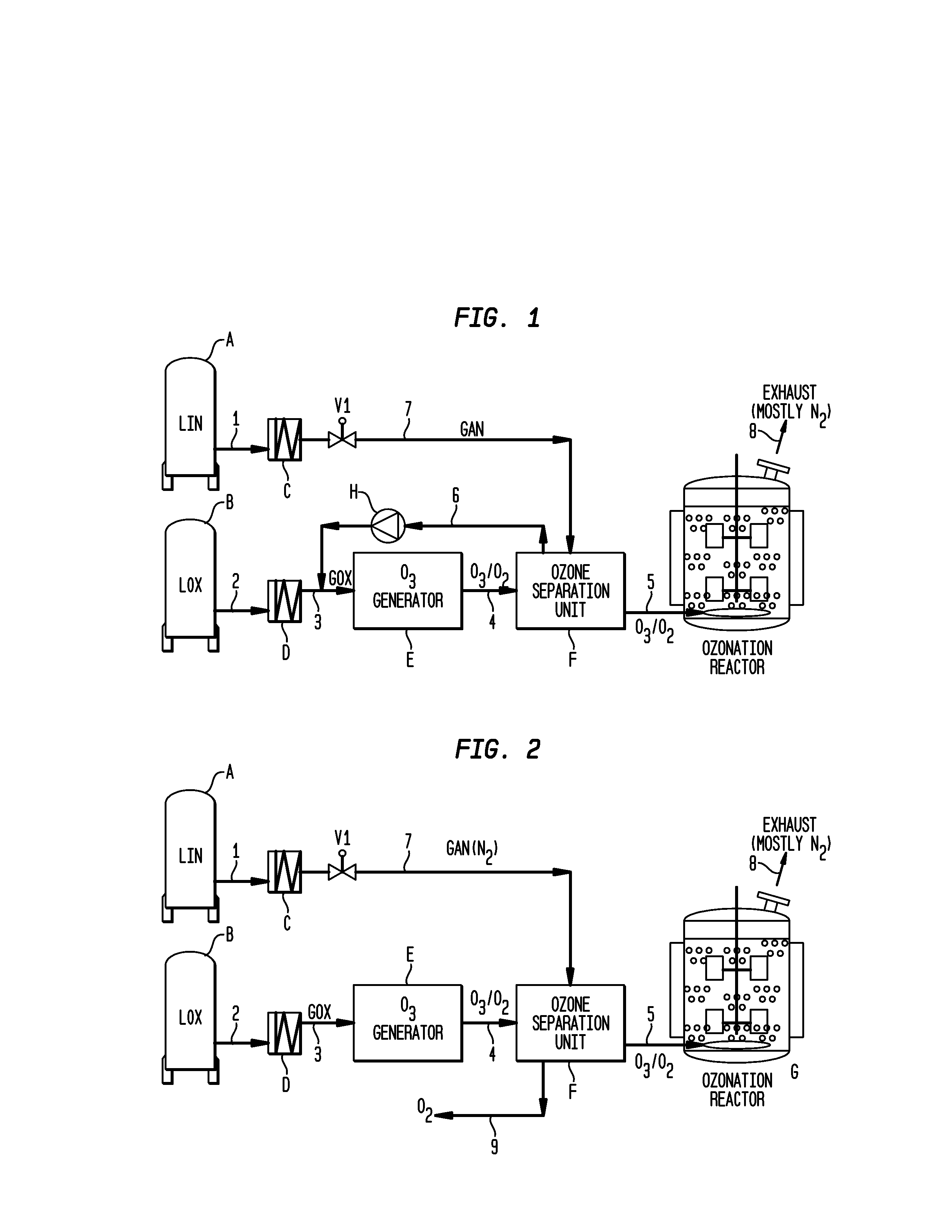
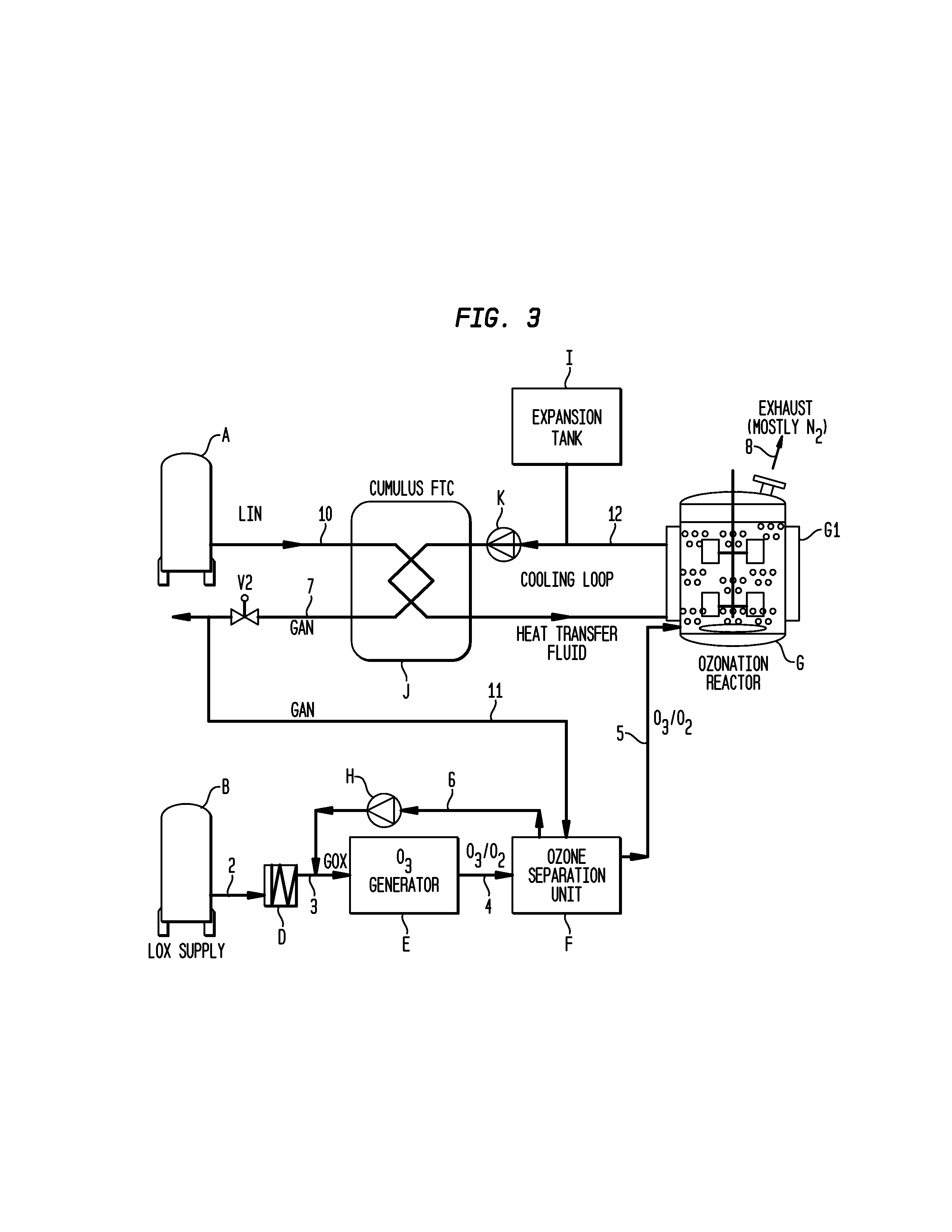
Methods for the ozonolysis of organic compounds
ActiveUS20130177497A1Low costAvoid increased oxygen levelsGas treatmentPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesSimple Organic CompoundsOzonolysis
A method for producing ozone for use in ozonolysis reactions. Oxygen is separated from the mixture of ozone and oxygen from an ozone generation unit and is fed back to the oxygen feed to the generation unit. Nitrogen is fed to the ozone separation unit and the mixture of nitrogen and ozone is fed to the ozonation reactor where the ozone will react with organic compounds to produce desired end products.
Owner:MESSER IND USA INC
Methods of making reversible crosslinked polymers and related methods
Methods are provided for making reversible crosslinked polymers. Exemplary methods comprise reacting first and second thermoplastic polymers having non-hindered olefins in the presence of a metathesis catalyst under conditions sufficient to form a crosslinked polymer. In certain embodiments, the methods comprise providing a crosslink promoting additive to improve the strength of the crosslinked polymer. In some embodiments, the methods comprise decrosslinking a crosslinked polymer through a metathesis or an ozonolysis reaction.
Owner:TE CONNECTIVITY CORP
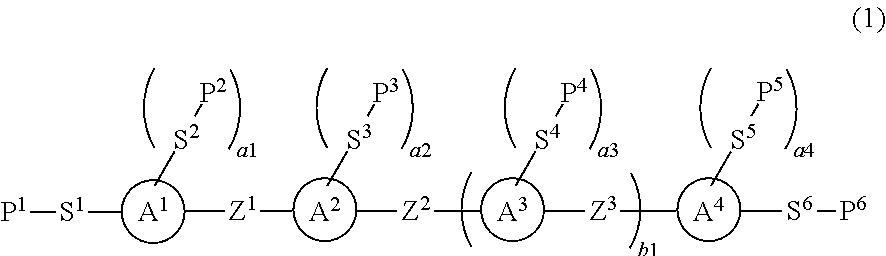
Polymerizable compound having triple bond, liquid crystal composition and liquid crystal display device
InactiveUS20150376505A1High polymerization reactivityWide temperature rangeLiquid crystal compositionsPreparation by ozonolysisPolymer chemistryMaterials science
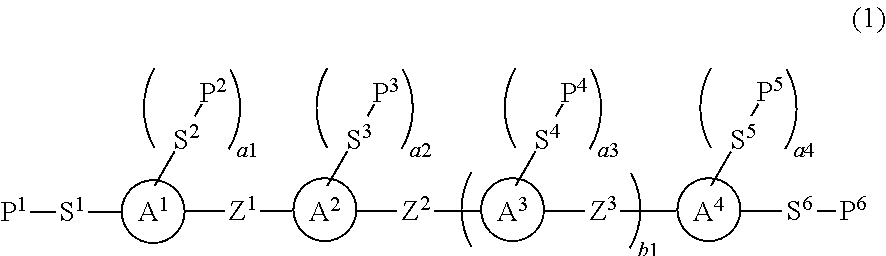
To provide a liquid crystal compound having high polymerization reactivity, a high conversion ratio and high solubility in a liquid crystal composition, a polymerizable composition containing the compound, a liquid crystal composite prepared using the composition, and a liquid crystal display device including the composite.The liquid crystal display device prepared using the polymerizable composition containing the compound represented by formula (1):wherein, in formula (1), P1 to P6 are a polymerizable group; S1 to S6 are a single bond, alkylene or the like; a1, a3 and a4 are an integer from 0 to 4, a2 is an integer from 1 to 4; ring A1 to ring A4 are a divalent group derived from benzene, naphthalene, anthracene, pyrimidine, pyridine or the like; Z1 to Z3 are a single bond, alkylene or the like, and at least one of Z1, Z2 and Z3 is —C≡C—; and b1 is 0 or 1.
Owner:JNC CORP +1
Toluene oxidation method
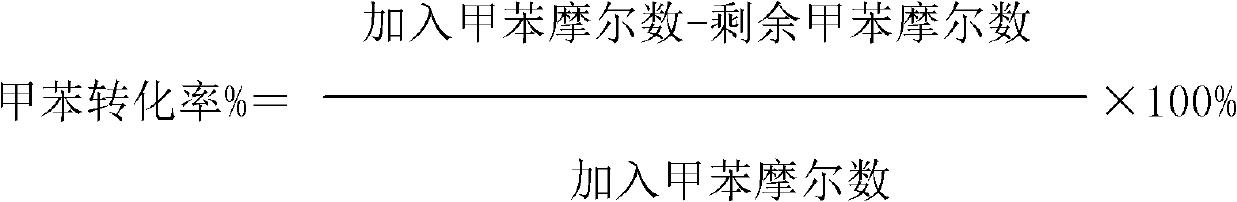
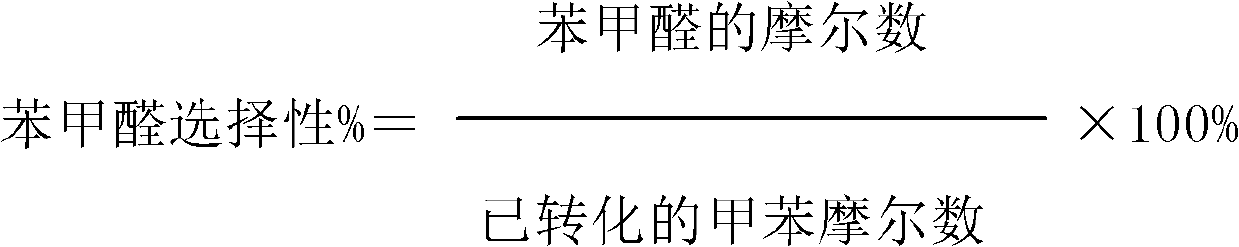
ActiveCN103288592AHigh selectivityImprove effective utilizationPreparation by ozonolysisOxygen compounds preparation by hydrocarbon oxidationBenzoic acidToluene oxidation
The invention discloses a toluene oxidation method. The method comprises a step of letting toluene make contact with an oxidizing agent under an oxidation reaction condition, and the method is characterized in that the oxidizing agent is a gas containing ozone. According to the invention, the total selectivity of benzaldehyde and benzoic acid is high, and the selectivity of benzaldehyde is improved in the presence of a catalyst containing titanium.
Owner:CHINA PETROLEUM & CHEM CORP +1
Natural benzaldehyde preparation method
The invention discloses a natural benzaldehyde preparation method, which comprises the following steps of: taking cinnamaldehyde as raw material; adding 0.5-10% of activated carbon catalyst modified by 0.1-1.0mol / L of hydrochloric acid; at the temperature of below-10-30DEG C, introducing in 0.05-1.0g of ozone at the rate of 1g of cinnamaldehyde per hour; carrying out ozonization reaction for 0.5-10 hours; under the condition of keeping the low temperature, dipping an ozonide intermediate into thiourea aqueous solution for reduction reaction; carrying out centrifugal separation; and finally carrying out molecular distillation at the temperature of 60DEG C and the pressure of 100Pa by a molecular distillation device to obtain the natural benzaldehyde with higher purity. The natural benzaldehyde preparation method disclosed by the invention has the advantages of simple technology, simplicity in operation, high reaction rate, high ozone use ratio, green and environmental-friendly reaction process, efficient catalyst, and is cheap and non-toxic and easy to separate from a product, the natural degree of the benzaldehyde can be kept, the selectivity of the benzaldehyde is good, and the purity and yield of the benzaldehyde are high.
Owner:GUANGXI UNIV
Method for preparing 1,6-adipaldehyde
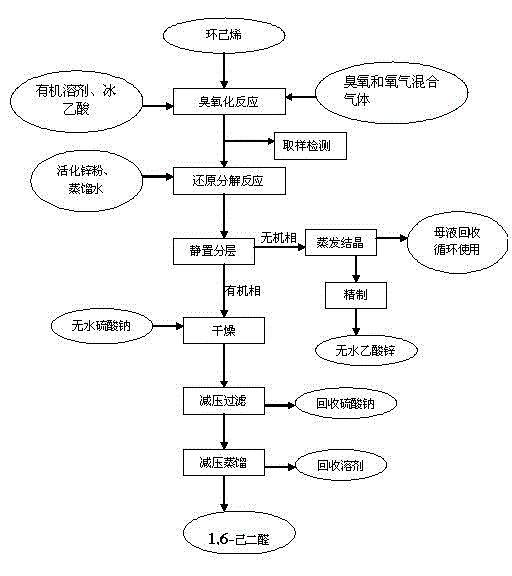
InactiveCN102746127AHigh activityGood choicePreparation by ozonolysisCarboxylic acid salt preparationCyclohexeneReaction temperature
The invention belongs to the field of synthesis of fine chemicals, and discloses a method for preparing 1,6-adipaldehyde. The method comprises the following steps of: performing an ozonization reaction on cyclohexene serving as a raw material by taking ozone as an oxidant and taking an organic solvent and glacial acetic acid as mixed solvents at the reaction temperature of between 20 DEG C below zero and 10 DEG C to obtain an ozonized reaction liquid, and directly performing a reducing reaction on an ozonide without separating; and reducing and decomposing by adopting zinc powder, and reacting under the protection of nitrogen gas at the room temperature for 0.5-1.5 hours to obtain 1,6-adipaldehyde. Compared with the conventional method, the method has the advantages: ozone is taken as an oxidant, so that high oxidizing capacity and high selectivity are realized, the environmental pollution is low, the requirement of ozonization reaction temperature is low, energy is saved, and consumption is lowered. After reacting, a product is easy and convenient to separate; and zinc acetate is recovered from a water phase by concentrating and crystalizing, so that production cost is lowered, and industrial production is facilitated.
Owner:中国平煤神马控股集团有限公司 +1
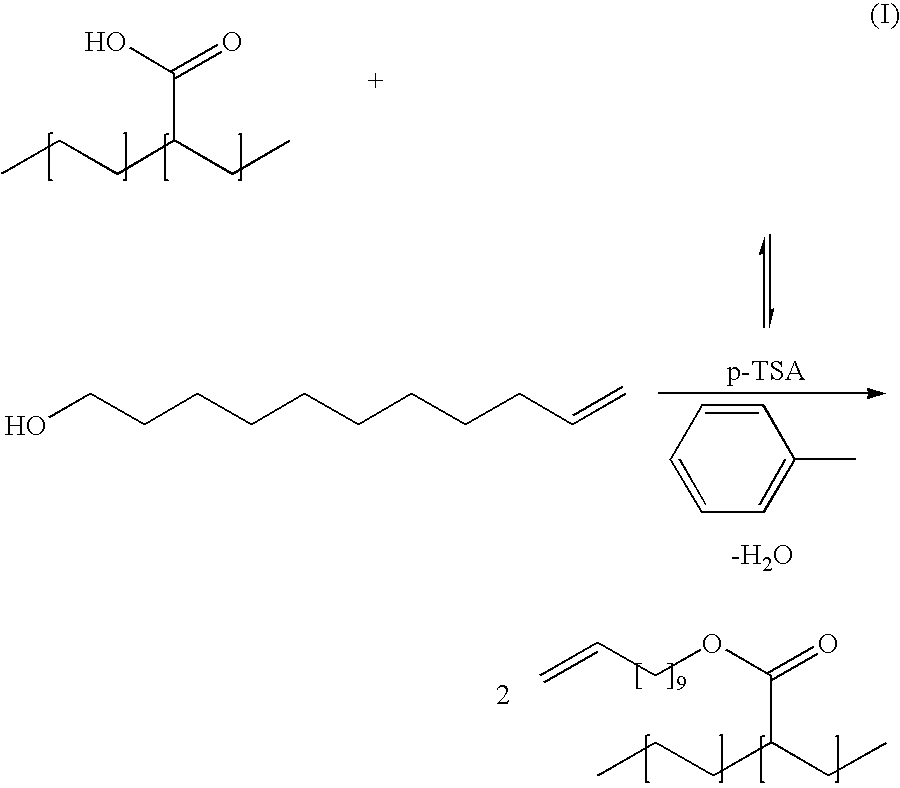
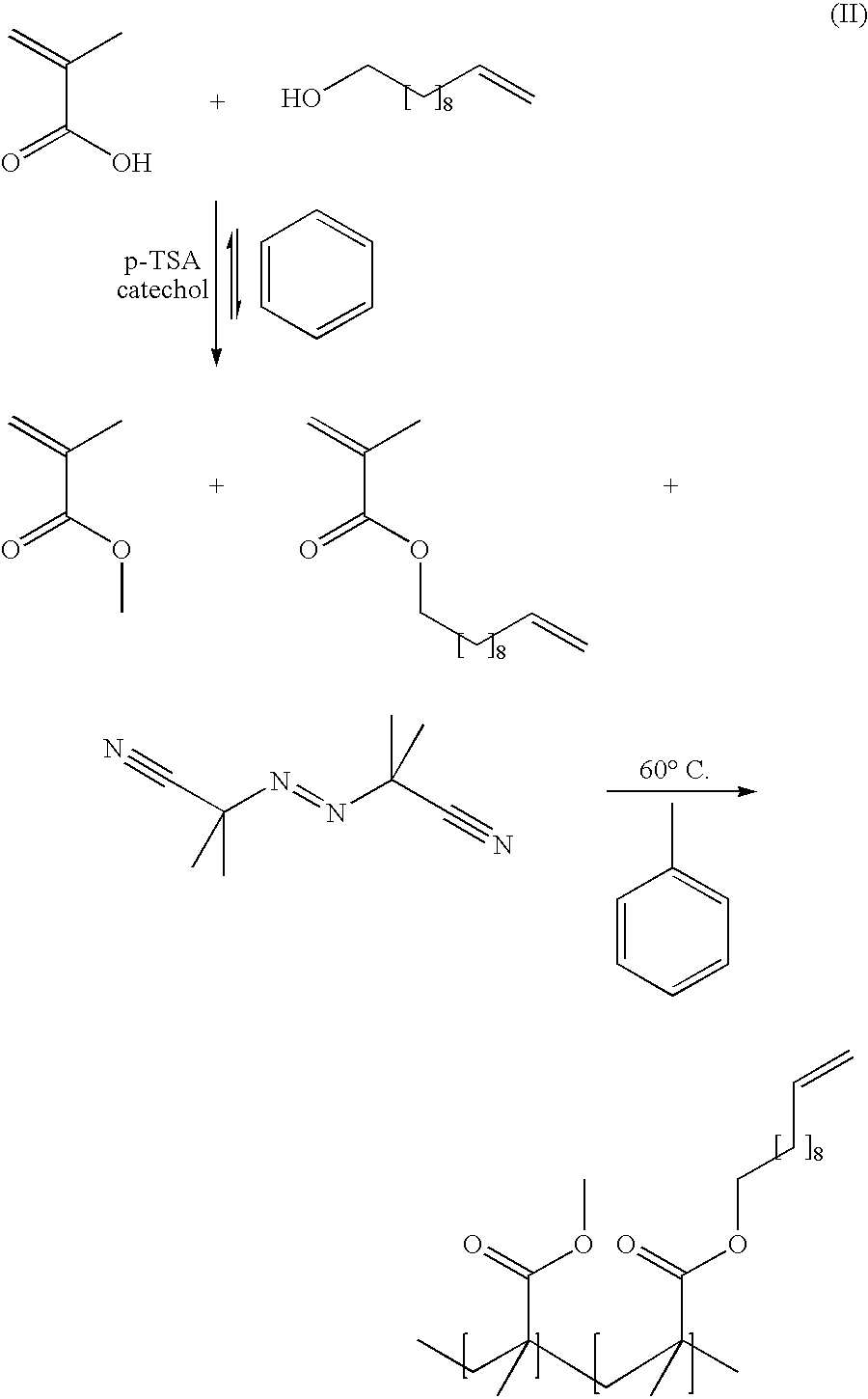
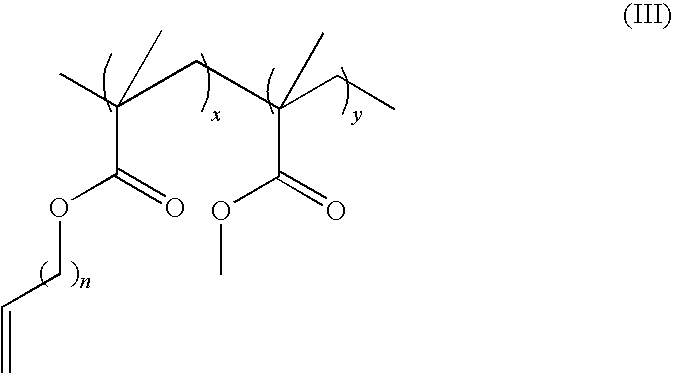
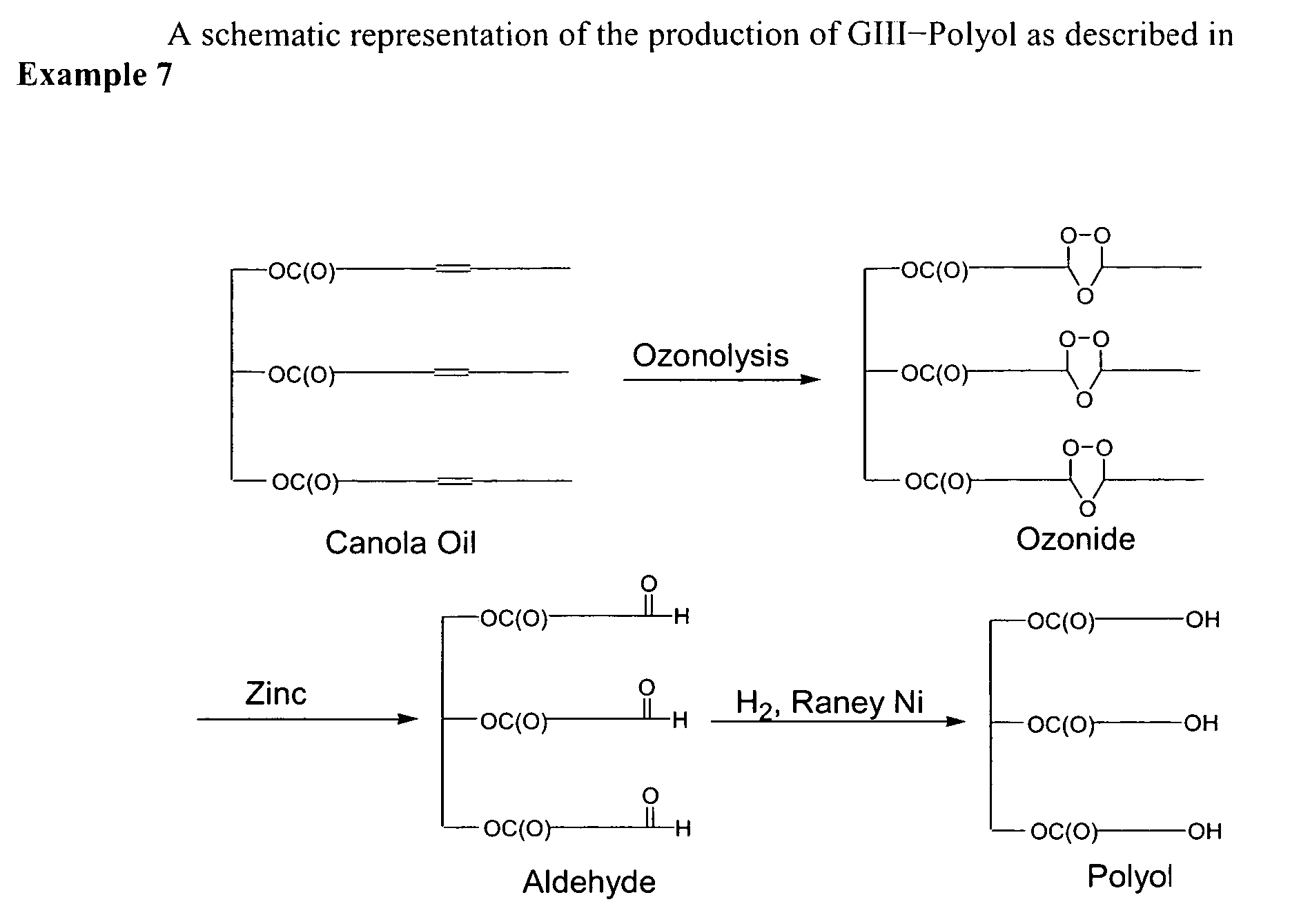
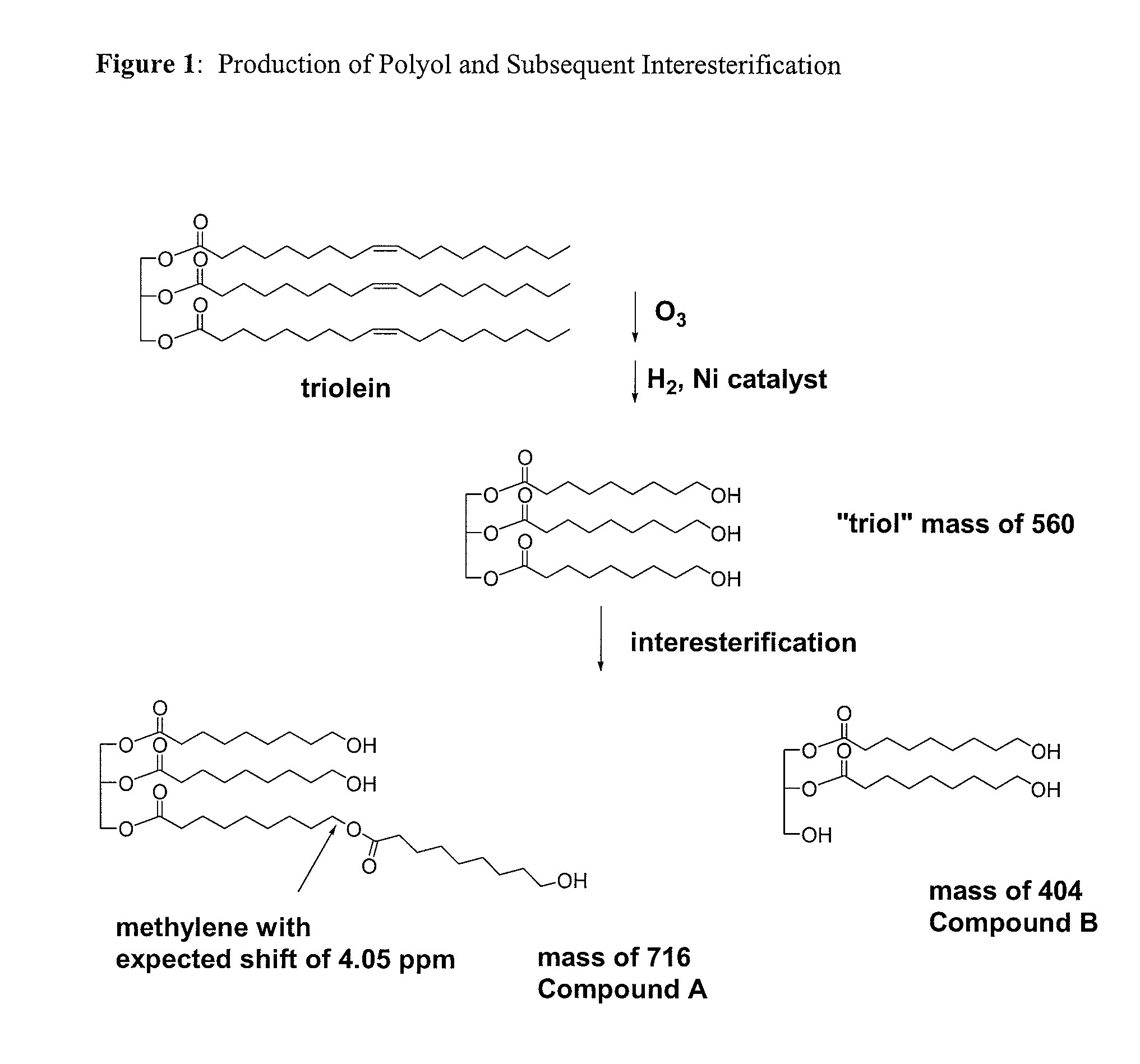
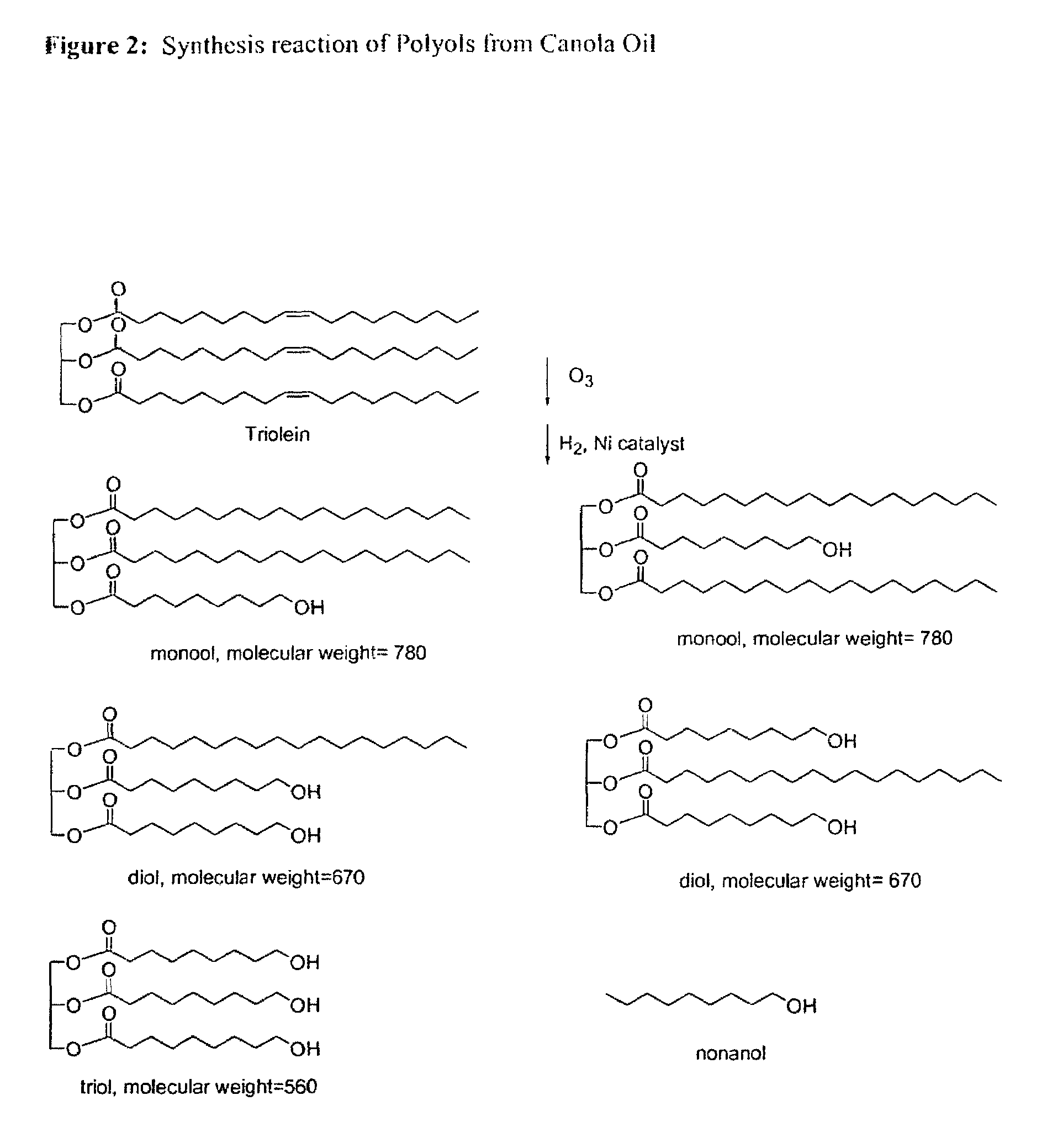
Bioplastics, monomers thereof, and processes for the preparation thereof from agricultural feedstocks
InactiveUS7538236B2Reducing steric hindrance to crosslinkingProduce some attenuationFatty acid hydrogenationCoke ovensAlcoholWax ester
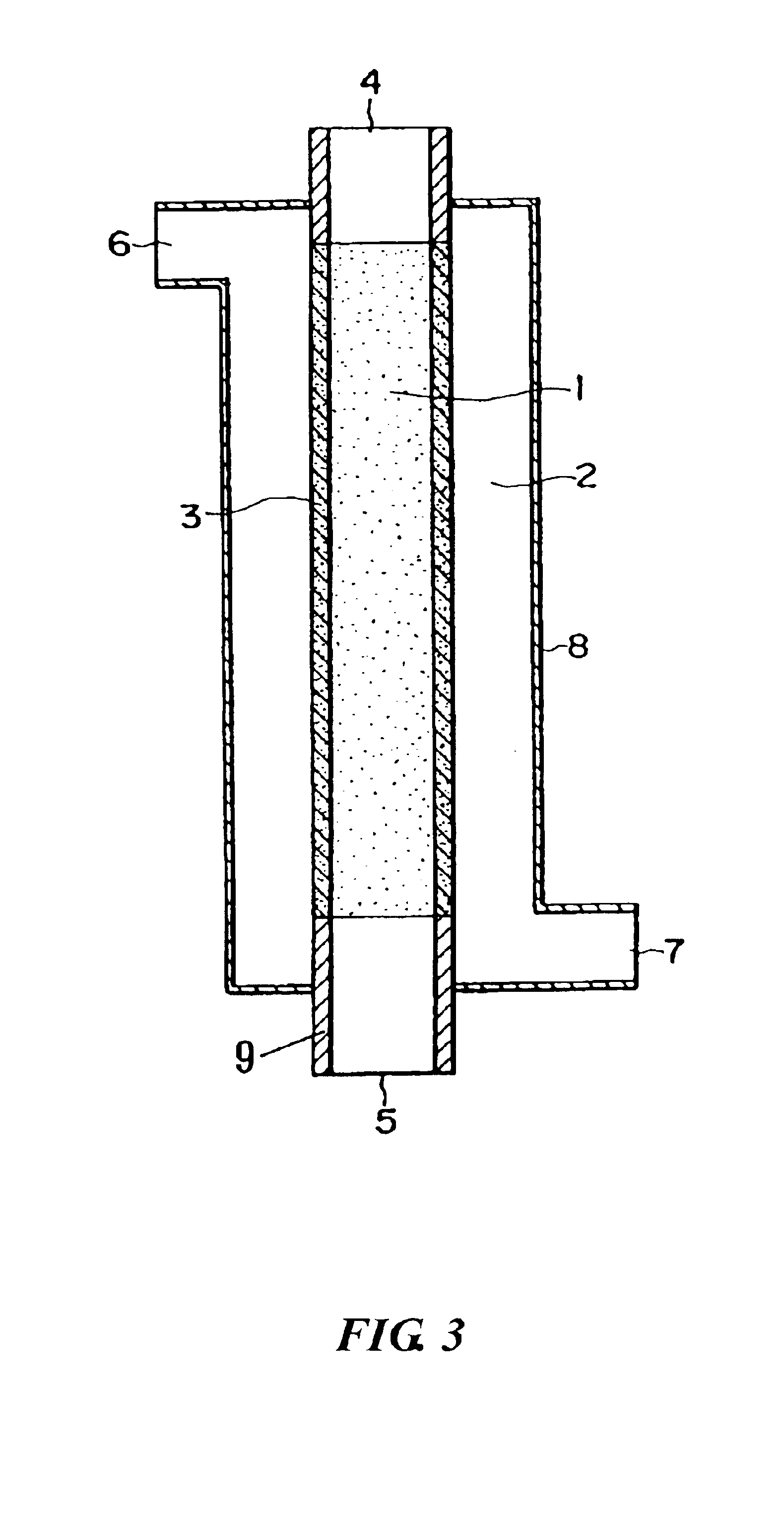
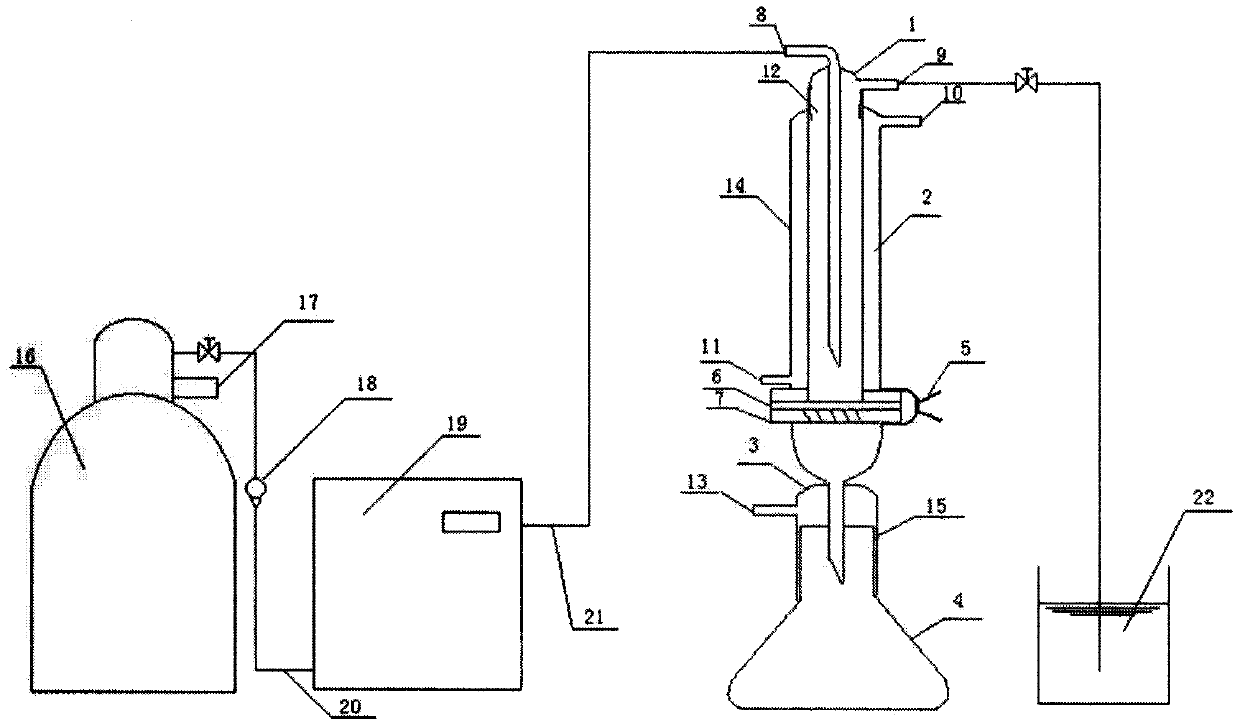
The present invention relates generally to polymers and monomers derived from agricultural feedstocks, and more particularly to methods for the production of monomers from renewable agricultural resources such as feedstocks, for example canola, flax and tallow, and polymers, in particular polyurethanes produced from monomers derived from such feedstocks. The present invention also relates to novel processes for the production of short-chain alcohols, as well as hydroxyl wax esters, from renewable feedstocks. An improved apparatus for carrying out ozonolysis reactions is also disclosed.
Owner:THE GOVERNORS OF THE UNIV OF ALBERTA
Preparation method of natural benzaldehyde by ozone collaborative heterogeneous catalysis of cinnamaldehyde or cinnamon oil
ActiveCN105601481AIncrease profitGood choicePreparation by ozonolysisMetal/metal-oxides/metal-hydroxide catalystsDistillationBenzaldehyde
The invention discloses a preparation method of natural benzaldehyde by ozone collaborative heterogeneous catalysis of cinnamaldehyde or cinnamon oil. The method is as below: adding 0.5%-10% of aNiO, BaO, MgO, La2O3, SnO2 binary or ternary oxide component catalyst into a raw material cinnamic aldehyde or cinnamon oil, introducing ozone in a bubble reactor under conditions of atmospheric pressure temperature of -10 to 30 DEG C and flow rate of 0.1-5 g / L, and reacting at low temperature for 1-8 h to obtain a benzaldehyde crude product; and finally, using a molecular distillation apparatus to obtain high-purity natural benzaldehyde. The invention has the advantages of simple process, convenient operation, no toxicity or harm of the catalyst, environment-friendliness; the cinnamaldehyde is fully utilized; and the benzaldehyde has good selectivity, high yield, and natural degree intact.
Owner:广西庚源香料有限责任公司
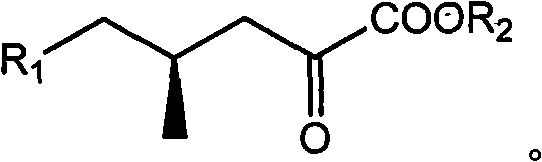
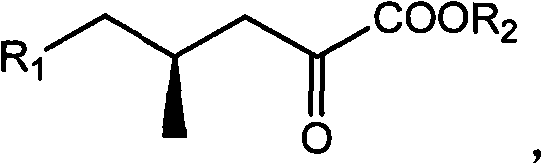
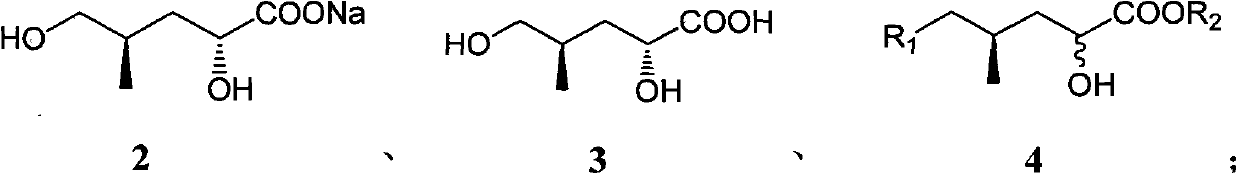
(4R)-4-methyl-2-carbonyl valerate compound, synthesizing method and application
InactiveCN102010336ASingle structureOrganic compound preparationCarboxylic acid esters preparationPotassiumMethyl group
The invention relates to a (4R)-4-methyl-2-carbonyl valerate compound with a methyl chiral functional group. The compound is prepared by converting waste (2R,4R)-2,5-dyhydroxyl-4-methyl potassium valerate which is oxidatively degraded by steroidsapogenin. The method is easy and convenient to operate, not only can improve the utilization rate of the steroidsapogenin, but also reduces pollution of waste of sterides degradation to the environment.
Owner:SHANGHAI INST OF ORGANIC CHEMISTRY - CHINESE ACAD OF SCI
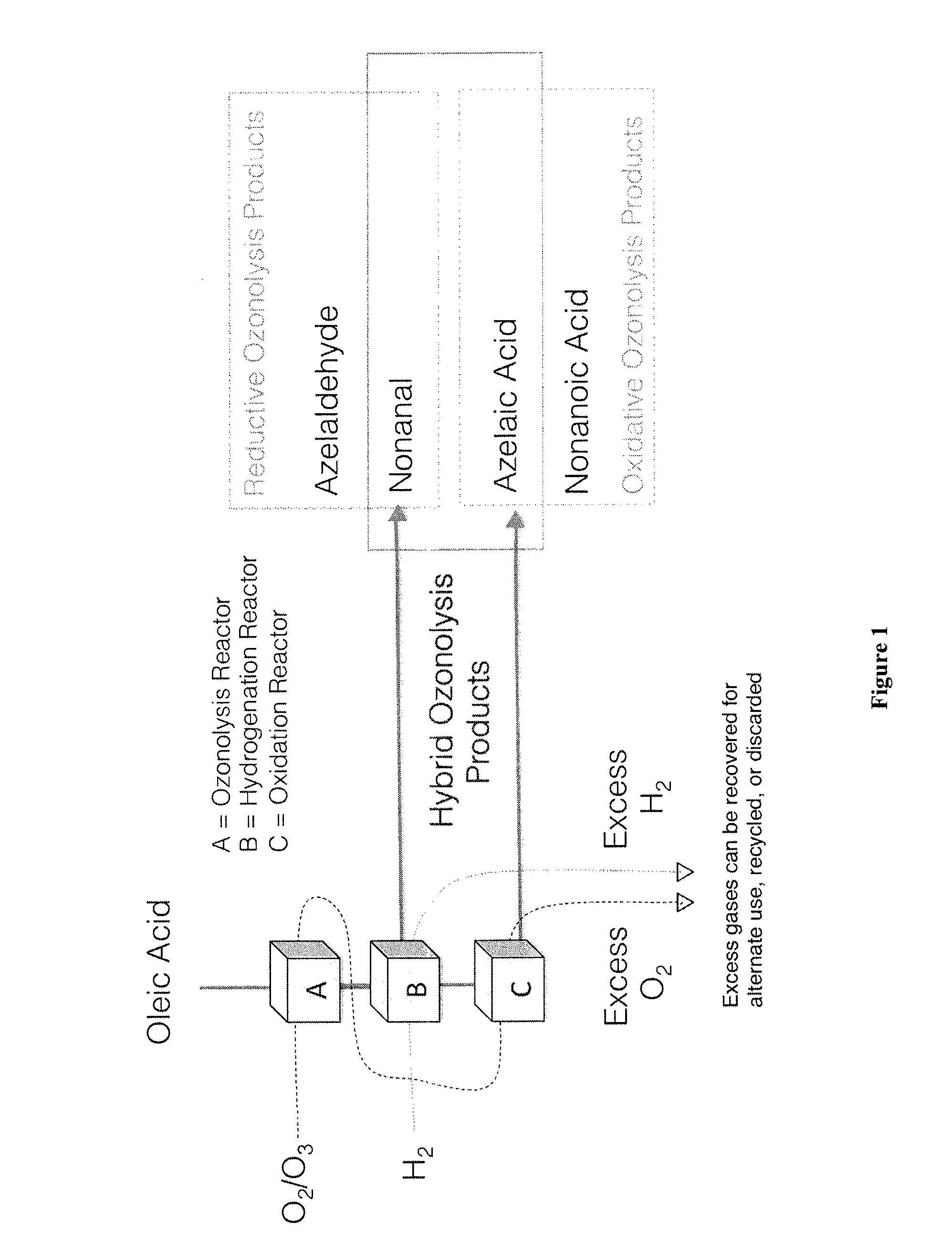
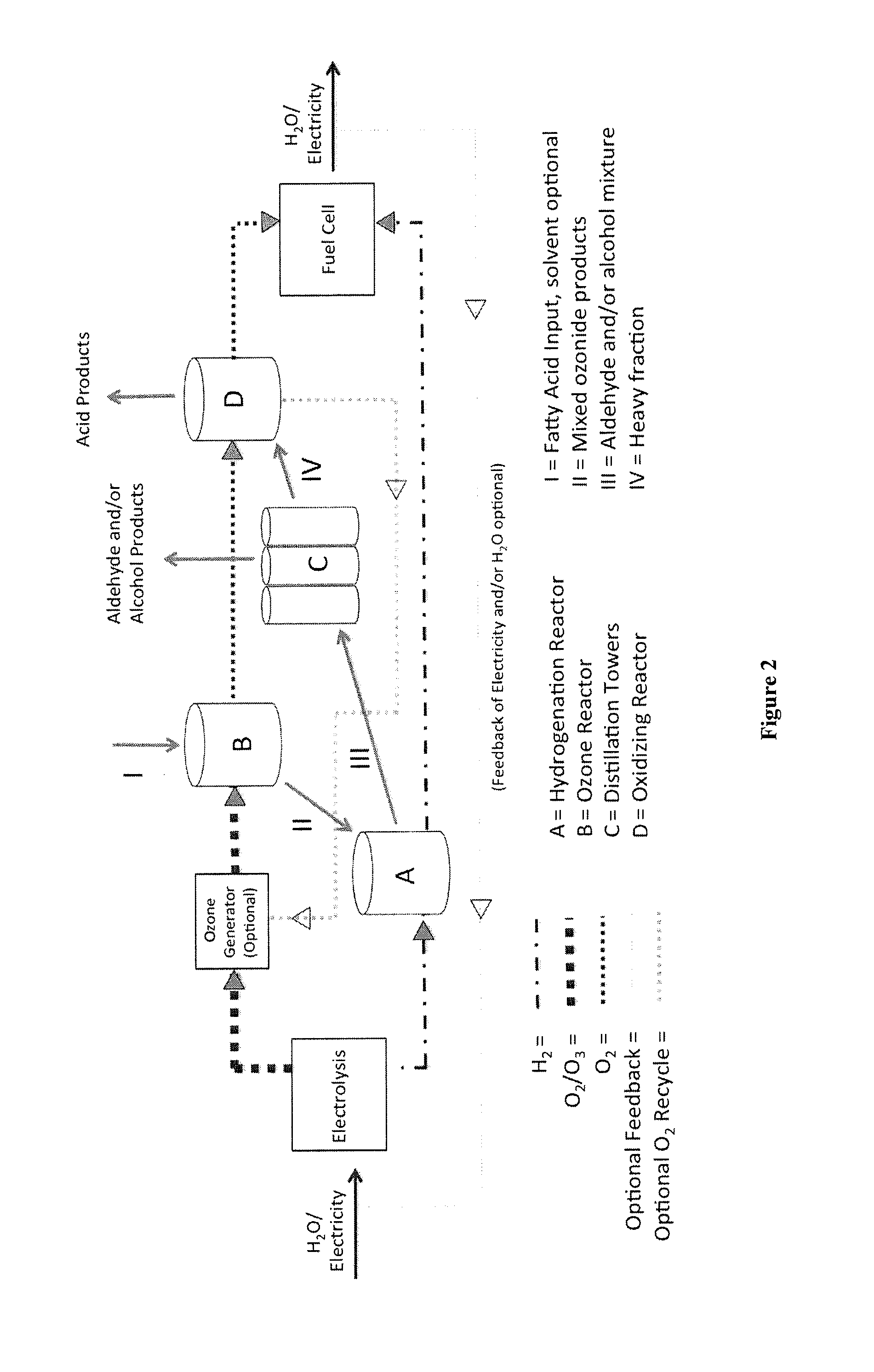
Ozonolysis operations for generation of reduced and/or oxidized product streams
ActiveUS9035091B2Minimization requirementsOrganic compound preparationPreparation by ozonolysisElectricityHydrogen
The present invention relates to methods for safe and efficient use of hydrogen and oxygen in ozonolysis operations. The invention also relates to an ozonolysis process involving elements of both reductive and oxidative ozonolysis which are integrated in a continuous process. In one embodiment, the ozonolysis process of the present invention uses hydrogen and / or oxygen generated from water and electricity, which may be recycled to generate water and / or electricity.
Owner:CONNECTICUT INNOVATIONS
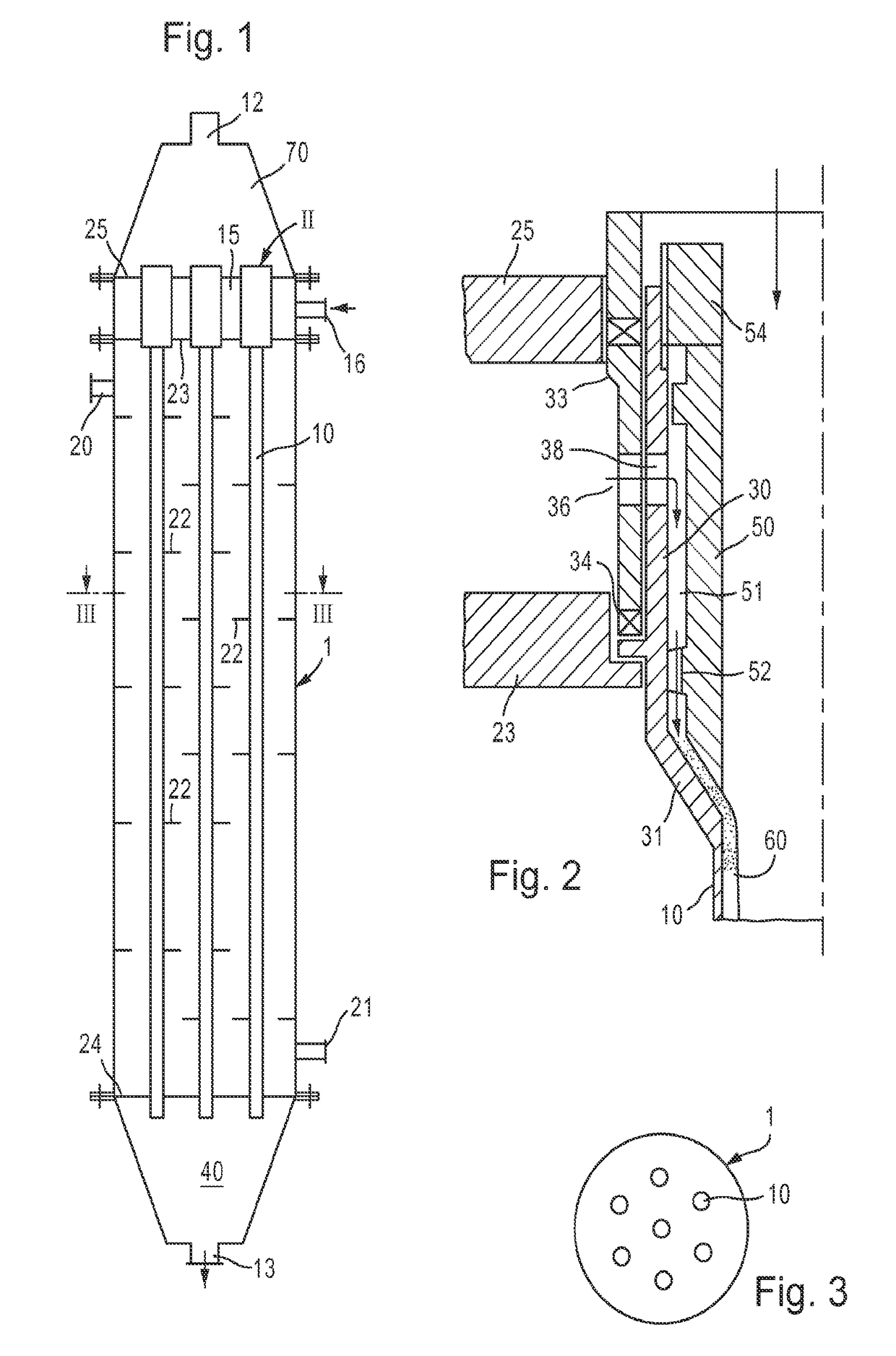
Film ozonolysis in a tubular or multitubular reactor
ActiveUS10071944B2Facilitated DiffusionImprove responseOrganic compound preparationWater contaminantsOzonolysisMembrane reactor
The disclosure relates to a method of performing ozonolysis or ozone-based oxidation on a liquid or emulsified reagent using a tubular falling film reactor with one or multiple tubes wherein the combined ozone and carrier gas flow is co-current.
Owner:P2 SCI INC
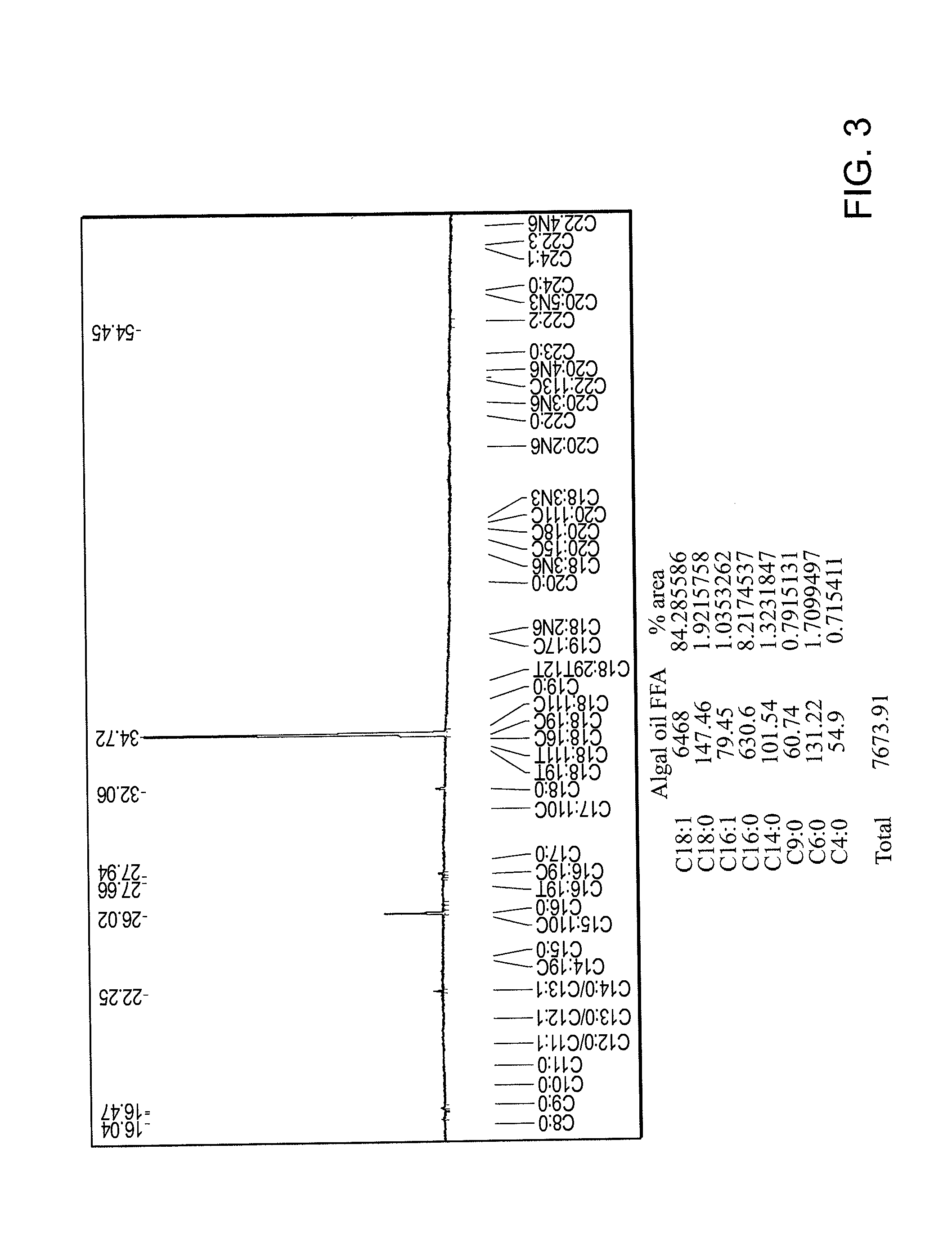
Ozonolysis of unsaturated fatty acids and derivatives thereof
ActiveUS20130078685A1High reaction yieldReduce appearance problemsOrganic compound preparationPreparation by ozonolysisOrganic solventOzonolysis
The invention relates to a method for the ozonolysis of unsaturated fatty acids and derivatives thereof, comprising a) ozonolysis of a fatty acid or of a derivative thereof in an organic phase comprising an organic solvent, and b) contacting the organic phase with an aqueous phase comprising catalase and preferably a buffer, where the fatty acid or the derivative thereof has a linear chain having at least eight carbon atoms, and the use of catalase for removing reactive oxygen species from a reaction mixture comprising ozonolysis products of an unsaturated fatty acid.
Owner:EVONIK OPERATIONS GMBH
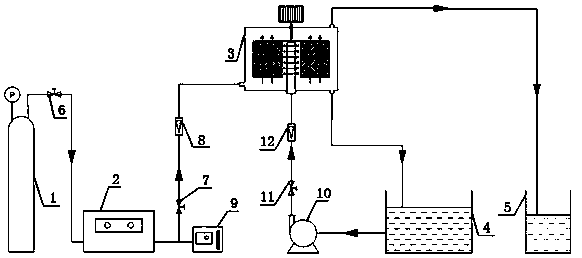
Preparation method of benzaldehyde from cinnamaldehyde by continuous catalytic ozone oxidation
The invention discloses a preparation method of benzaldehyde from cinnamaldehyde by continuous catalytic ozone oxidation, which comprises the following steps: by using cinnamaldehyde or cassia oil as the raw material, adding a mixed solution of 50g cinnamaldehyde and anhydrous ethanol into a continuous ozonization reactor, adding 0.5-5.0% of one or more of MnO2, ZnO, Fe2O3 and CaO as a catalyst, introducing 0.05-0.85g of ozone to every gram of cinnamaldehyde per hour at -5-20 DEG C to carry out ozonization reaction for 10 minutes, continuously introducing the cinnamaldehyde-anhydrous ethanol mixed solution at the rate of 50-100g.h<-1>.gcat<-1> to continuously react to obtain a liquid-phase product, and distilling the liquid-phase product in a molecular distillation device at certain temperature under certain pressure to obtain the higher-purity benzaldehyde. The method has the advantages of simple technique, green reaction process, favorable selectivity and high benzaldehyde yield, can maintain the nature of the benzaldehyde, and can implement continuous production of natural benzaldehyde.
Owner:GUANGXI UNIV
Ozonolysis for activation of compounds and degradation of ozone
Provided is an inactive compound that is activated by reaction with ozone into an active compound having a carbonyl oxygen. Also provided is a method of activating the above inactive compounds. Further provided is a method of treating a disease or condition in a subject using the above compound at a site that is not exposed to atmospheric ozone. Additionally provided is a method of determining internal ozonolysis in a subject using the above compound. Also provided is a molecule less than 1000 mw, having a double bond that is reactive with ozone, and forms a nontoxic compound after reacting with ozone. Further provided is a method of degrading ozone.
Owner:AIR CROSS
Method for oxidizing styrene
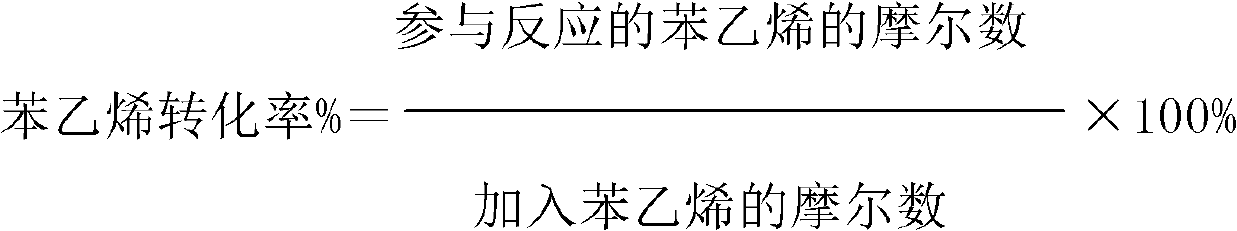
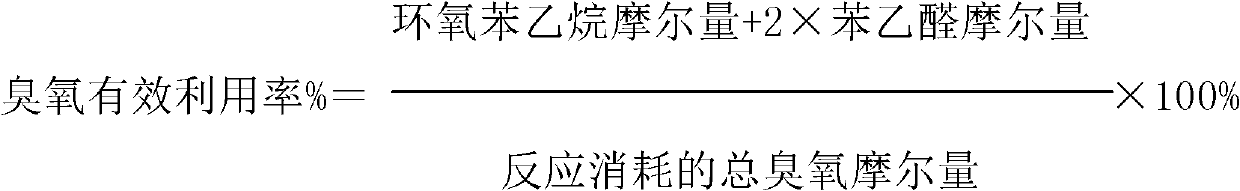
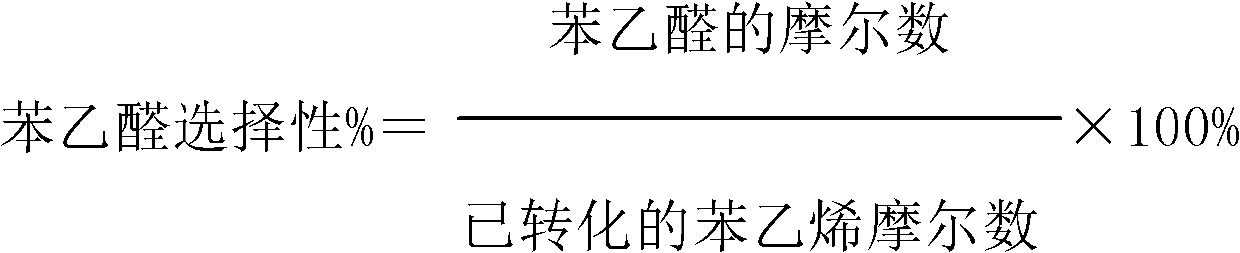
ActiveCN103288611AImprove effective utilizationHigh selectivityPreparation by ozonolysisStyrene oxideBenzene
The invention discloses a method for oxidizing styrene. According to the method, the styrene comes into contact with an oxidant under the condition of oxidation reaction. The method is characterized in that the oxidant is an ozone-containing gas. According to the method, the phenylacetaldehyde selectivity is high, and the selectivity of styrene oxide is greatly improved in the presence of a titanium-containing catalyst.
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparation method of benzaldehyde
ActiveCN104311405AGood choiceHigh purityPreparation by ozonolysisCarbonyl compound separation/purificationPhoto catalyticPhotocatalytic reaction
The invention discloses a preparation method of benzaldehyde. The method comprises the following steps: by taking cinnamyl aldehyde or cinnamyl oil as a raw material, adding 1.0-5.0% of one or more photocatalysts such as TiO2, ZnO, SnO2, Bi2O3, Y2O3 and the like; putting in an inner radiating photo-catalytic reactor; introducing 0.03-0.5g of ozone into per gram of cinnamyl aldehyde per hour at -5-20 DEG C for ozone synergized photo-catalytic reaction for 0.5-5 hours to obtain a coarse benzaldehyde product; and finally, obtaining benzaldehyde with relatively high purity by virtue of a molecular distilling device. The method disclosed by the invention is simple in process and green in reaction process, and the nature degree of benzaldehyde can be maintained. Moreover, the method is good in selectivity and high in benzaldehyde yield.
Owner:SUN YAT SEN UNIV
Method for preparing anisaldehyde
InactiveCN101792378AImprove solubilityImprove antioxidant capacityPreparation by ozonolysisSulfite saltDistillation
The invention discloses a method for preparing anisaldehyde. The method comprises the following steps: (1) mixing anethole, organic solvents and water to obtain reaction materials; adding the reaction materials into a bubble reaction tower and introducing mixed gas of ozone and oxygen to carry out ozonization reaction; continuing introducing oxygen to expel redundant ozone after the ozonization reaction is over to obtain ozonization reaction liquid; and (2) heating anhydrous sodium sulfite solution to 60-80 DEG C and dropwise adding the ozonization reaction liquid to carry out reduction reaction; continuing reacting for 30-60min after dropwise adding the ozonization reaction liquid, cooling and standing the reactant and dividing the liquid to obtain the coarse anisaldehyde; and removing the organic solvents by distillation to obtain the anisaldehyde product. The method avoids a little side reaction in the process of ozonization, lowers the equipment and production cost, solves the keyproblems confronted by industrialized production, such as equipment corrosion due to presence of acetic acid, heavy environmental pollution and the like, and opens up a way for large-scale industrialized production.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
Preparation method of benzaldehyde
InactiveCN101250096AInhibit aggregationAvoid reactionOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by ozonolysisOrganic solventBenzaldehyde
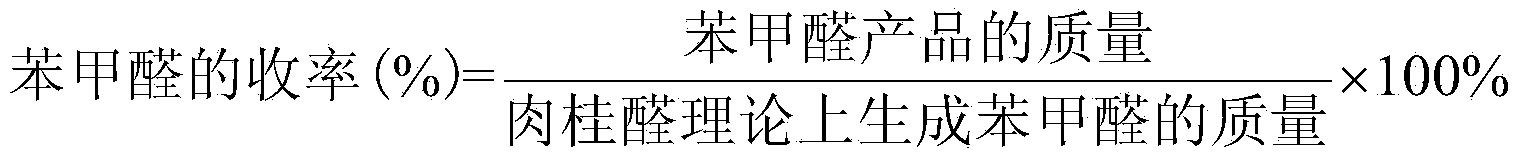
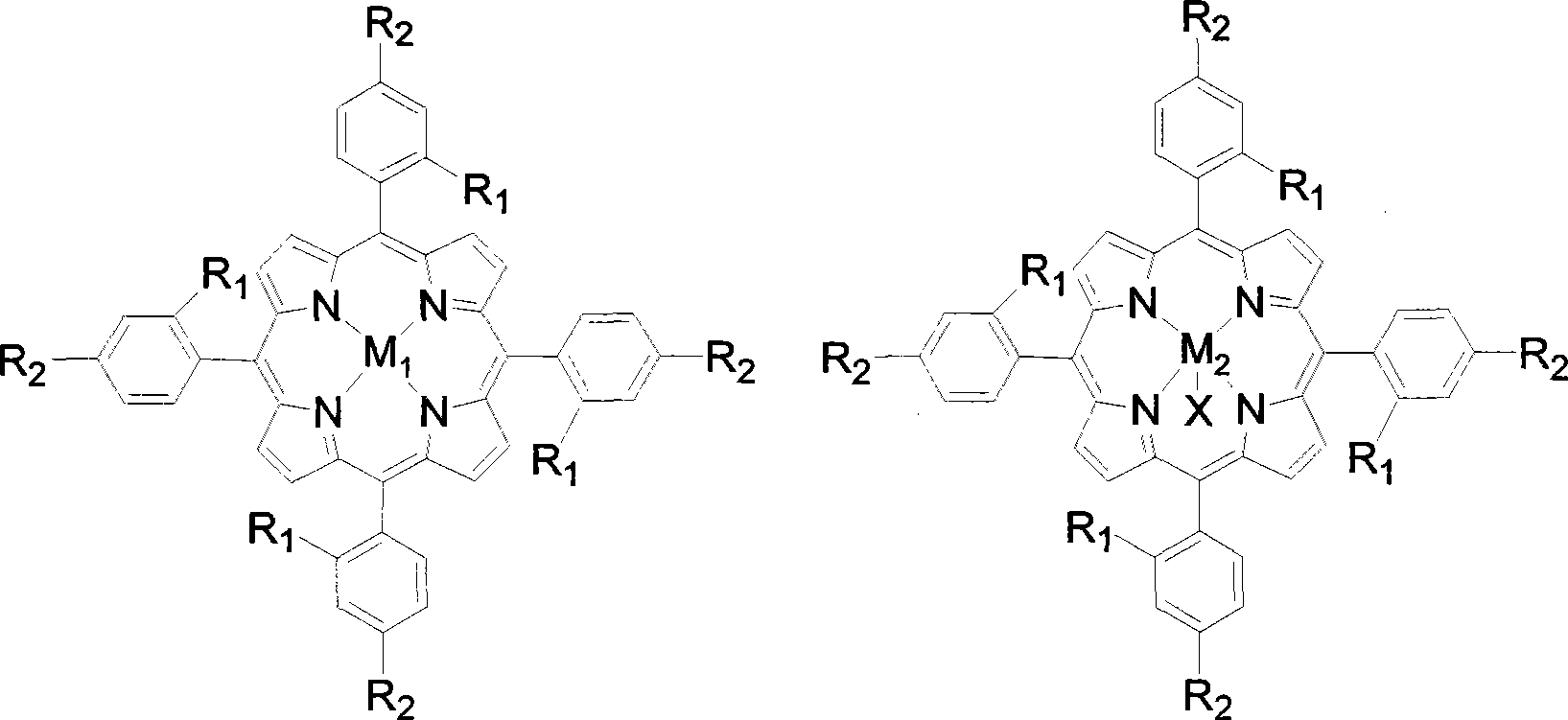
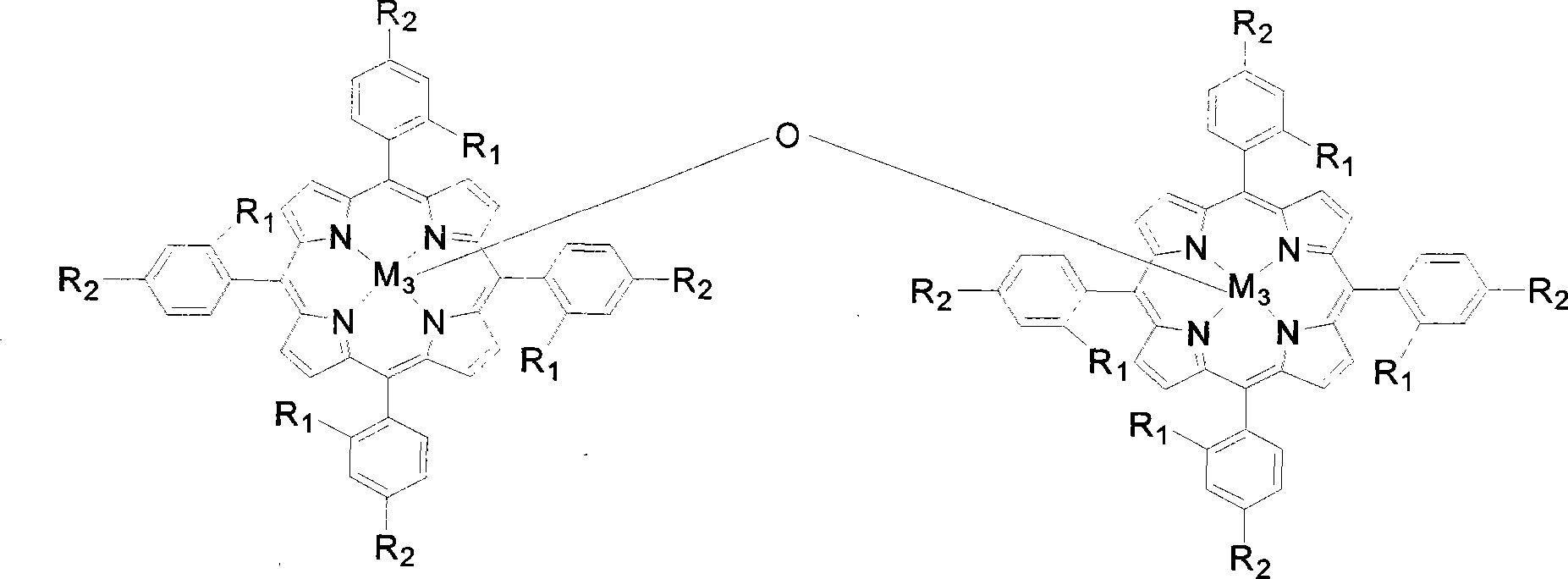
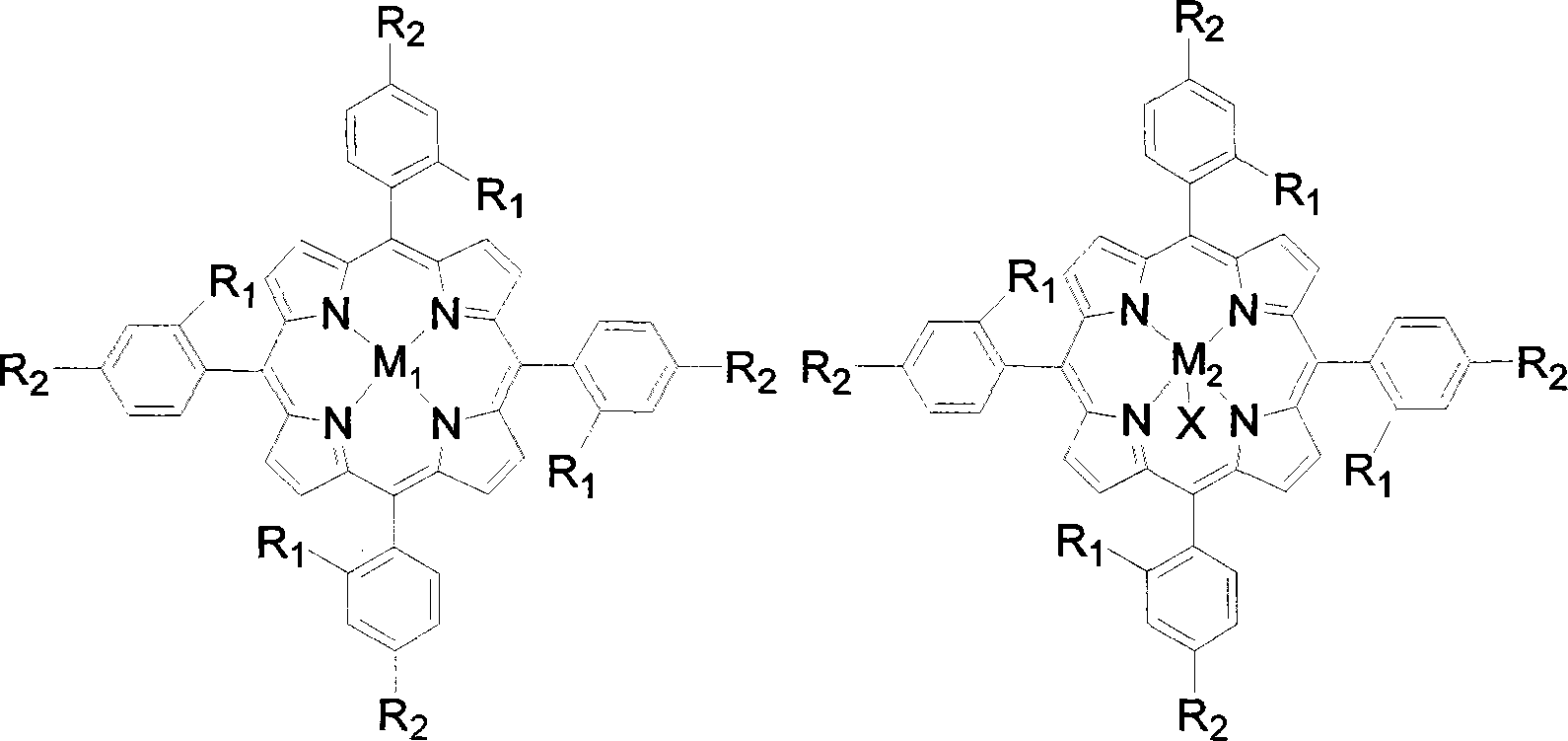
The invention discloses a preparation method of benzaldehyde, which uses cinnamaldehyde or cinnamon oil as raw material, dissolves the materials in organic solvent and uses metalloporphyrin compound as catalyst, adds oxidant, controls the reaction temperature at 20-100DEG, and reacts for 2-12h to obtain benzaldehyde. The preparation method of benzaldehyde has the advantages of mild reaction conditions, low catalyst consumption, better catalysis effect, simple process, high product yield and better selectivity.
Owner:SUN YAT SEN UNIV
Natural benzaldehyde preparation method
The invention discloses a natural benzaldehyde preparation method, which comprises the following steps of: taking cinnamaldehyde as raw material; adding 0.5-10% of activated carbon catalyst modified by 0.1-1.0mol / L of hydrochloric acid; at the temperature of below-10-30DEG C, introducing in 0.05-1.0g of ozone at the rate of 1g of cinnamaldehyde per hour; carrying out ozonization reaction for 0.5-10 hours; under the condition of keeping the low temperature, dipping an ozonide intermediate into thiourea aqueous solution for reduction reaction; carrying out centrifugal separation; and finally carrying out molecular distillation at the temperature of 60DEG C and the pressure of 100Pa by a molecular distillation device to obtain the natural benzaldehyde with higher purity. The natural benzaldehyde preparation method disclosed by the invention has the advantages of simple technology, simplicity in operation, high reaction rate, high ozone use ratio, green and environmental-friendly reaction process, efficient catalyst, and is cheap and non-toxic and easy to separate from a product, the natural degree of the benzaldehyde can be kept, the selectivity of the benzaldehyde is good, and the purity and yield of the benzaldehyde are high.
Owner:GUANGXI UNIV
Cyclohexanol oxidation method
ActiveCN102850198AHigh selectivityImprove conversion ratePreparation by ozonolysisCarboxylic preparation by ozone oxidationCyclohexanol oxidationOxidizing agent
The invention provides a cyclohexanol oxidation method, which includes allowing cyclohexanol to contact an oxidant under oxidation reaction conditions, wherein the oxidant is ozone-containing gas. The inventive method can oxidize cyclohexanol into cyclohexanone in the absence of catalyst by using ozone as oxidant, with simple process and high selectivity of target product.
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparation method of natural benzaldehyde
InactiveCN102826978BGood choiceHigh purityPreparation by ozonolysisMetal/metal-oxides/metal-hydroxide catalystsBenzaldehydeDistillation
The invention discloses a preparation method of natural benzaldehyde. The method comprises the following steps of: getting cinnamyl aldehyde or cinnamon oil as a raw material; adding one or more of multi-phase catalysts of 0.5% to 10% of MnO2, TiO2, Al2O3, SnO2, Fe2O3, MgO, CuO, CeO2, ZrO2, Bi2O3, Y2O3 or active carbon; pouring 0.05 to 0.5g of ozone in a bubbling reactor at -5 to 20 DEG C based on 1g of cinnamyl aldehyde per hour; carrying out an ozonization reaction for 0.5 to 10 hours to obtain an ozonide intermediate; dropping the ozonide intermediate into the thiourea aqueous solution to be reduced while agitating at a constant low temperature, so as to obtain an oil-water mixture; separating the oil from the water to obtain a rough benzaldehyde product; and finally operating a molecular distillation device to obtain the benzaldehyde with relatively high purity. The preparation method has the advantages of simple technology, green reaction, being capable of remaining the natural property of the benzaldehyde, high selectivity, and high yield of the benzaldehyde.
Owner:GUANGXI UNIV
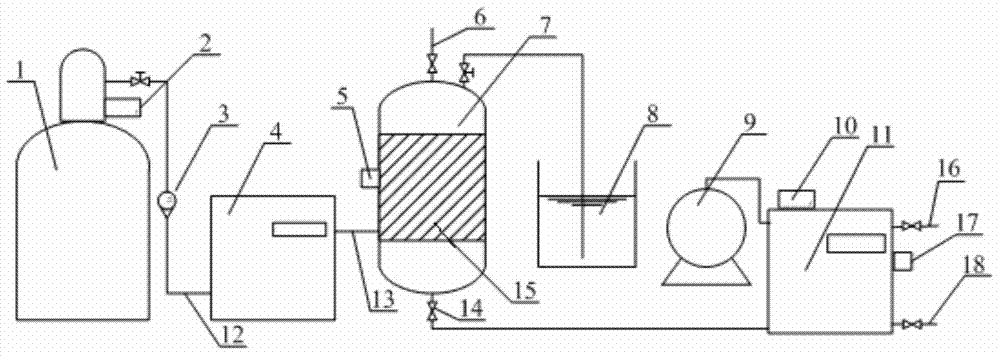
Supergravity reaction device and application thereof
ActiveCN110026145AAtom economy is highEnhanced MicromixingPreparation by ozonolysisCarboxylic preparation by ozone oxidationBenzoic acidOrganic synthesis
The invention discloses a supergravity reaction device and application thereof, belongs to the technical field of fine organic synthesis, and solves the problems existing in existing preparation processes of benzaldehyde and benzoic acid. According to the invention, fluid is greatly sheared and crushed through a supergravity rotating packed bed and a high-speed rotating packing, a huge and rapidlyupdating phase interface is generated, and the microcosmic mixing and mass transfer process is greatly enhanced. Ozone is selected as an oxidizing agent, and the device has the advantages of greenness, high efficiency, no secondary pollution and the like. Low-toxicity low-boiling-point reagents such as dichloromethane and ethyl acetate are selected as solvents, and recycling is facilitated. The method has the advantages of being environmentally friendly, high in atom economy and the like, and has wide industrial application prospects.
Owner:ZHONGBEI UNIV
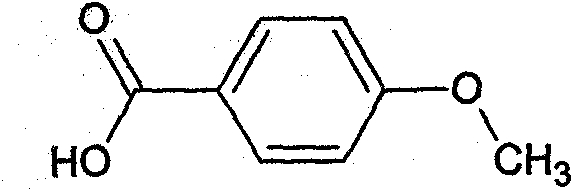
Preparation method of anisic acid
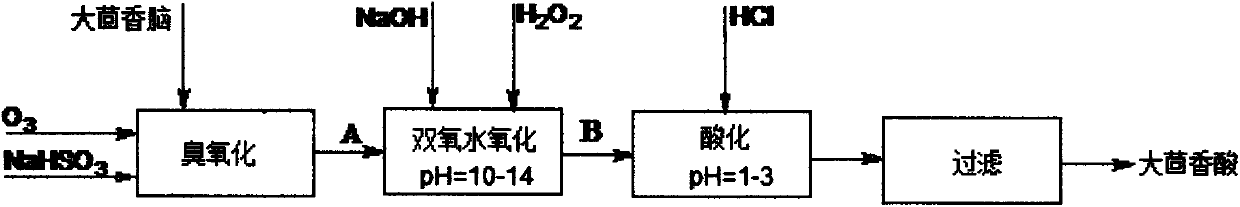
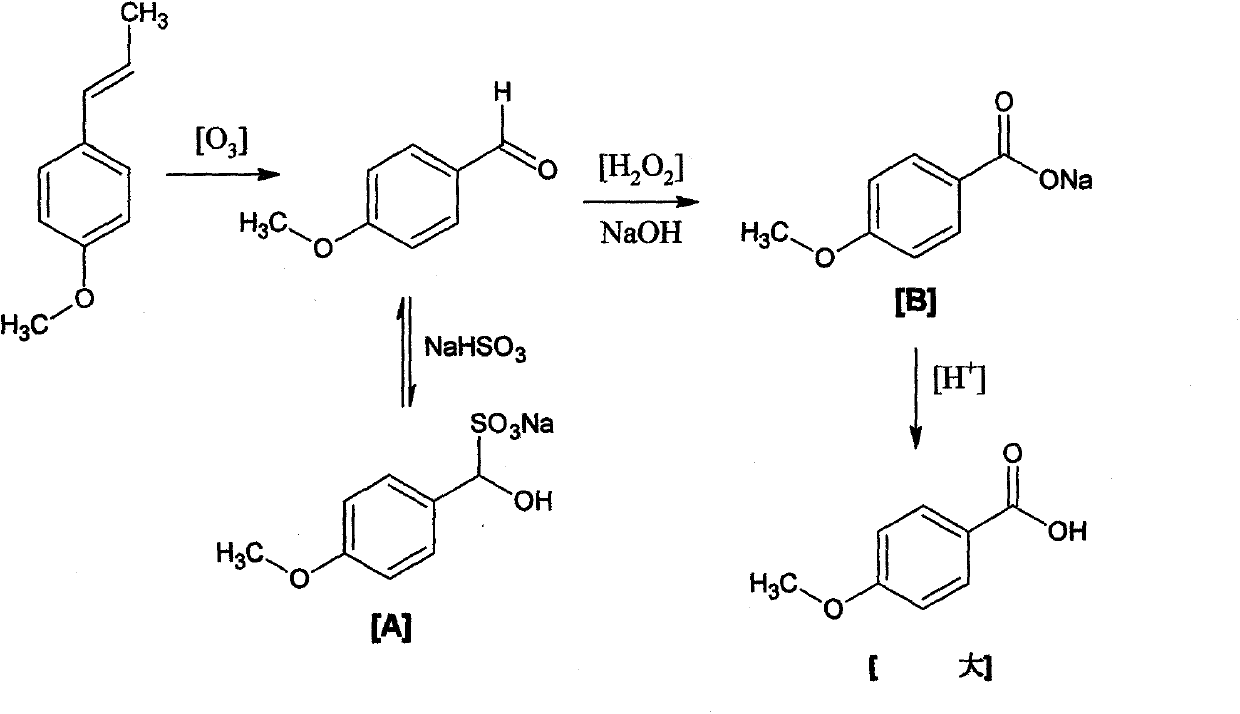
InactiveCN104177245AHigh yieldHigh purityPreparation from carboxylic acid saltsEther separation/purificationSulfite saltTurpentine
The invention discloses a preparation method of anisic acid, which comprises the following steps: taking a right amount of crude sulfate turpentine, and fractionating to obtain crush anethole; carrying out crystallizing separation on the anethole to obtain the high-purity anethole; adding the high-purity anethole and a NaHSO3 solution into a reaction kettle, and introducing ozone to perform oxidation for 110 minutes, thereby obtaining an anisic aldehyde sodium sulfite addition product A; simultaneously and slowly adding a sodium hydroxide solution and oxydol into A, stirring to react for 5.0-9.0 hours while keeping at 65-85 DEG C to obtain a reaction product B; and after the reaction is finished, adding a HCl solution into B to regulate the pH value to 1-3, and filtering to obtain the anisic acid. The method has the advantages of high yield and high purity and does not use any organic solvent in the separation process, so the product has no toxic or harmful solvent residue and can be used in the industries of medicine, food and cosmetics; the method has the characteristics of mild reaction conditions, simple purification and refinement and high product purity, and is simple to operate; and by using the ozone and oxydol as oxidizers, the reaction product is water, so the oxidizers are green and environment-friendly.
Owner:广西辰康生物科技有限公司
Catalytic process for the preparation of isolongifolene
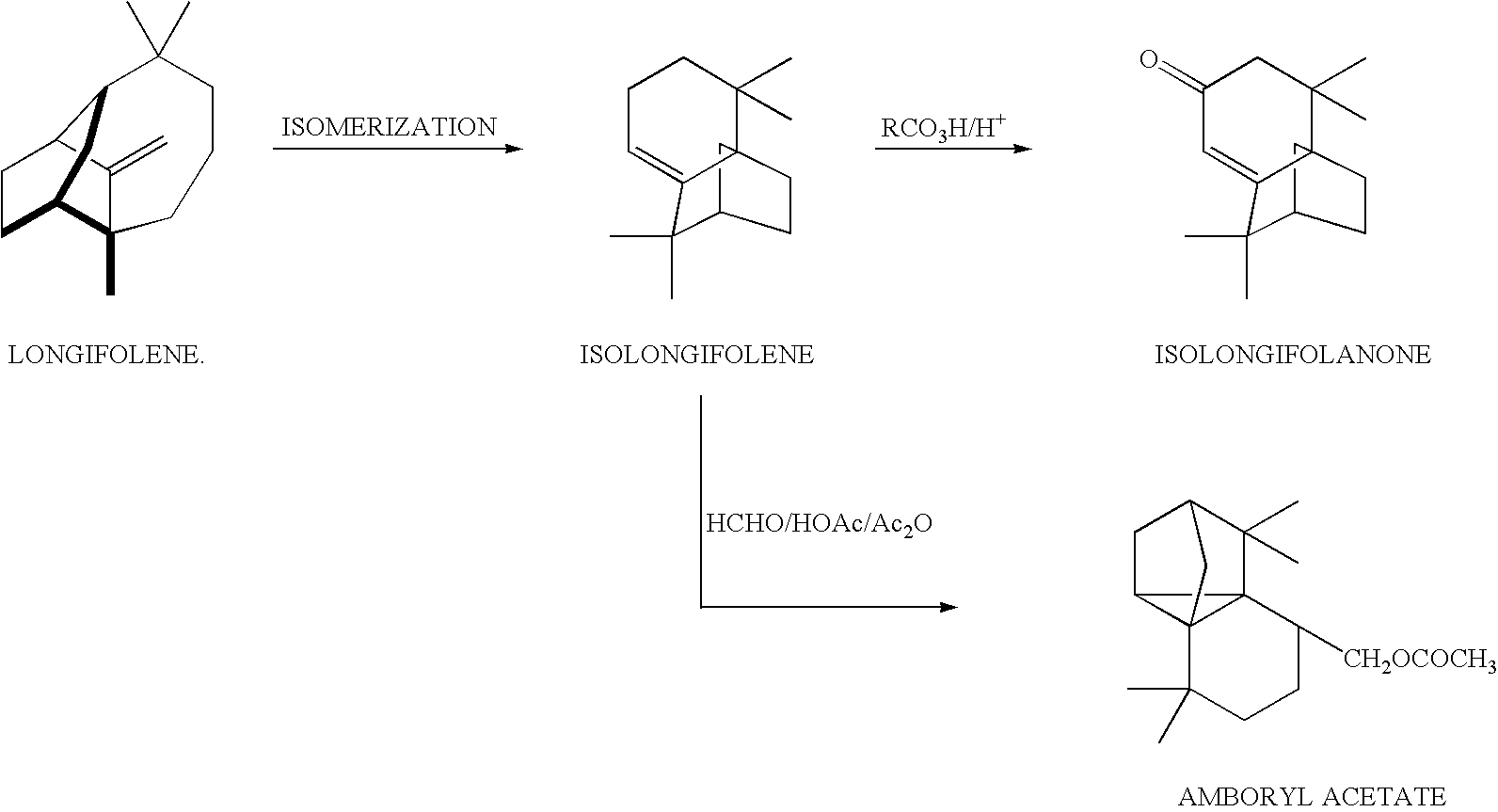
InactiveUS20040242936A1Speed up the conversion process% selectivityHydrocarbon by isomerisationFilter regenerationLongifoleneSolvent free
The present invention relates to a catalytic process for preparation of isolongifolene using nanocrystalline solid super acid. This process is an eco-friendly, single step, solvent free catalytic process for the preparation of a tricyclic sesqui-terpene hydrocarbon, isolongifolene. More particularly, the present invention provides a process for the catalytic isomerisation of longifolene to iso-longifolene using nano-crystalline sulfated zirconia as a solid super acid catalyst.
Owner:COUNCIL OF SCI & IND RES
Catalytic oxidation method of cyclohexane
InactiveCN104292090AHigh selectivityEasy to produceOrganic compound preparationPreparation by ozonolysisCyclohexanoneCatalytic oxidation
The invention discloses a catalytic oxidation method of cyclohexane for preparation of cyclohexanone. According to the method, at the temperature of 0-180 DEG C and at the pressure of 0.1-3.0 MPa, cyclohexane, an oxidizing agent, a solvent and a catalyst are mixed and contacted with each other to carry out a reaction, wherein the oxidizing agent is pure ozone or a gas mixture of pure ozone and diluent gas. The method provided by the invention has higher cyclohexane conversion rate and cyclohexanone selectivity.
Owner:王晓伟
Reaction method utilizing diaphram type catalyst and apparatus therefor
InactiveUS6911563B2High yieldEasy accessCatalytic gas-gas reactionOxygen-containing compound preparationCarboxylic acidKetone
A method for carrying out a reaction of one substance capable of being activated by a catalyst with another substance capable of reacting with said one substance activated, characterized in that the substance capable of being activated is activated by passing the substance through a diaphragm type catalyst and the reaction is thus performed in one reaction step; a method for producing an aromatic alcohol utilizing the above method; and a reaction apparatus suitable for these reactions. In the method, one substance is activated by passing through a diaphragm type catalyst and an objective reaction is carried out by using the activated substance, and the reaction can be performed in one reaction step and with safety. Moreover, the contact of the above activated substance with a compound to be reacted therewith can be freely controlled, and therefore, over-reaction can be prevented and an objective product can be produced in high yield. The method is thus markedly advantageous from an economical view point as a commercial process for producing oxygen-containing organic compounds such as an aromatic alcohol, a ketone, an aldehyde, a carboxylic acid and an epoxide.
Owner:NAT INST OF ADVANCED IND SCI & TECH +1
Method for preparing vanillin through sodium isoeugenol process
InactiveCN104086390AEasy to useEasy to preparePreparation by ozonolysisChemical industrySulfite salt
A method for preparing vanillin through a sodium isoeugenol process relates to the technical field of the chemical industry. The method comprises the following steps: mixing sodium isoeugenol and water, adding the obtained mixture into an oxidation reaction kettle, carrying out an oxidation reaction, connecting with a vent pipe to input ozone, increasing the ozone rate to drive the stirring and overturning of a liquid in the oxidation reaction kettle in order to carry out a liquid phase oxidation reaction, layering the liquid obtained after the liquid phase oxidation reaction, adding a sodium sulfite solution to decompose ozonides, neutralizing the ozonides, washing with water to obtain crude vanillin, adding to a refining kettle, carrying out refining crystallization, drying to obtain finished vanillin, packaging and warehousing. The method has the advantages of convenient and simple preparation, environmental protection, no pollution, less equipment investment, high purity and convenient operation, and the prepared vanillin has the advantages of good use effect, safety and reliability.
Owner:安徽佑骏商品混凝土有限公司
Preparation method of benzaldehyde
InactiveCN109761773ASeparation is direct and fastHigh purityPreparation by ozonolysisCarbonyl compound separation/purificationBenzaldehydeDistillation
The invention discloses a preparation method of benzaldehyde. The preparation method comprises the steps that cinnamyl aldehyde serves as raw materials, a mixed solution of 20.0 g of cinnamyl aldehydeand absolute ethyl alcohol is loaded into an ozonization reactor, the mass ratio of the cinnamyl aldehyde to the absolute ethyl alcohol is 1:4, 3.0wt% of CaO is added as a catalyst, 0.05-0.95 g of ozone is introduced into per gram of cinnamyl aldehyde per hour at the temperature of minus 10 DEG C, and the ozonization reaction is conducted for 0.5-12.0 hours; while the reaction is conducted, a reaction product is subjected to membrane filter dehydration in a dewatering device of an ozonization reacting device at the pressure of 0.001-1.0 MPa through one of a PVA organic membrane, a NaA-type zeolite molecular sieve membrane, a T-type zeolite molecular sieve membrane, and a CHA-type zeolite molecular sieve membrane, thus the reaction is conducted in the forward direction, the high benzaldehyde yield is obtained, the obtained product is subjected to molecular distillation treatment, and then the natural benzaldehyde with the purity reaching 85% or above can be obtained. The preparation method has the advantages that the process is novel, operation is easy, the reaction process is environmentally friendly, the natural degree of the benzaldehyde is good, and the obtained benzaldehyde ishigh in purity and yield.
Owner:GUANGXI UNIV
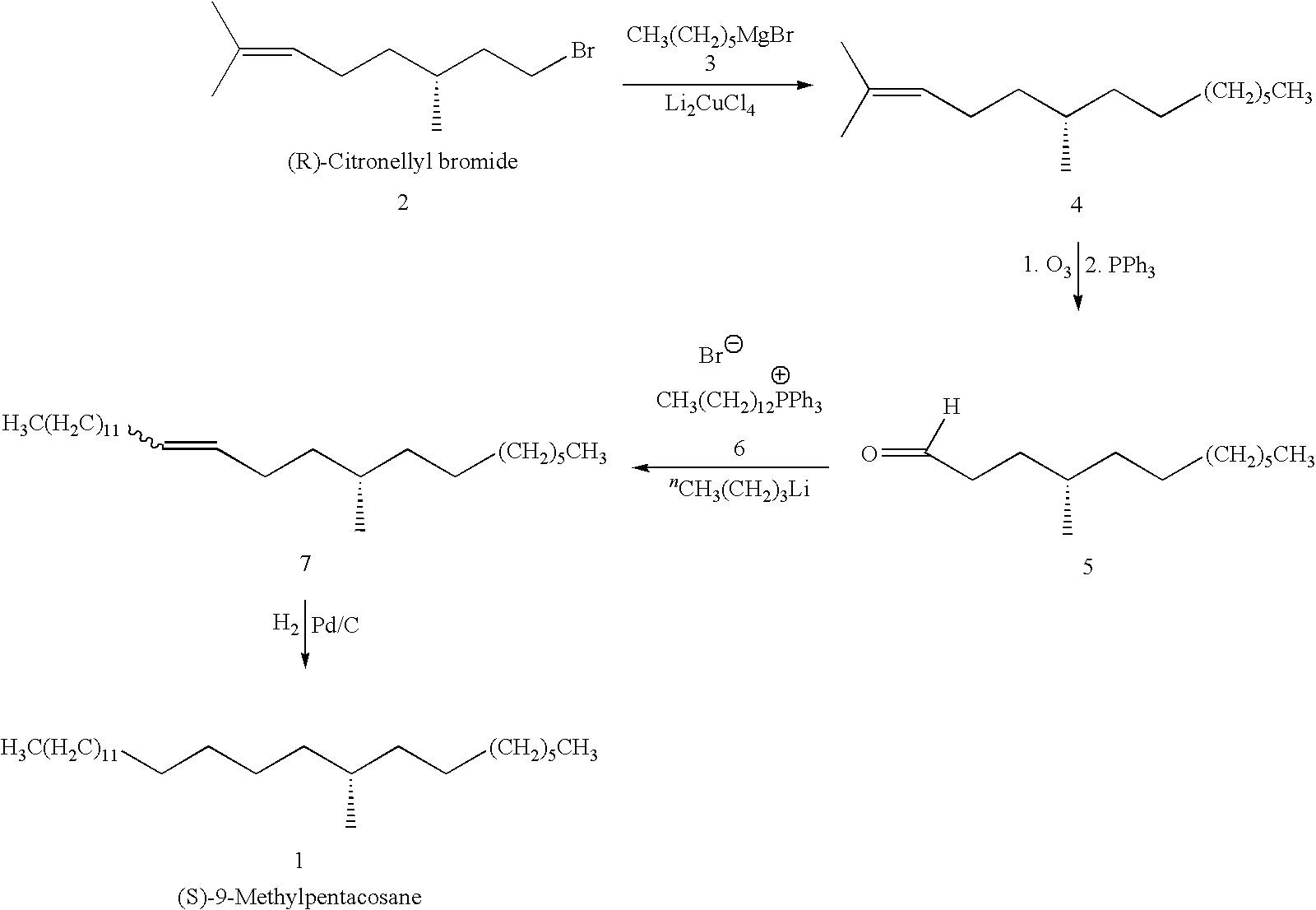
Contact sex pheromone component of the Emerald Ash Borer Agrilus planipennis Fairmaire (Coleoptera: Buprestidae)
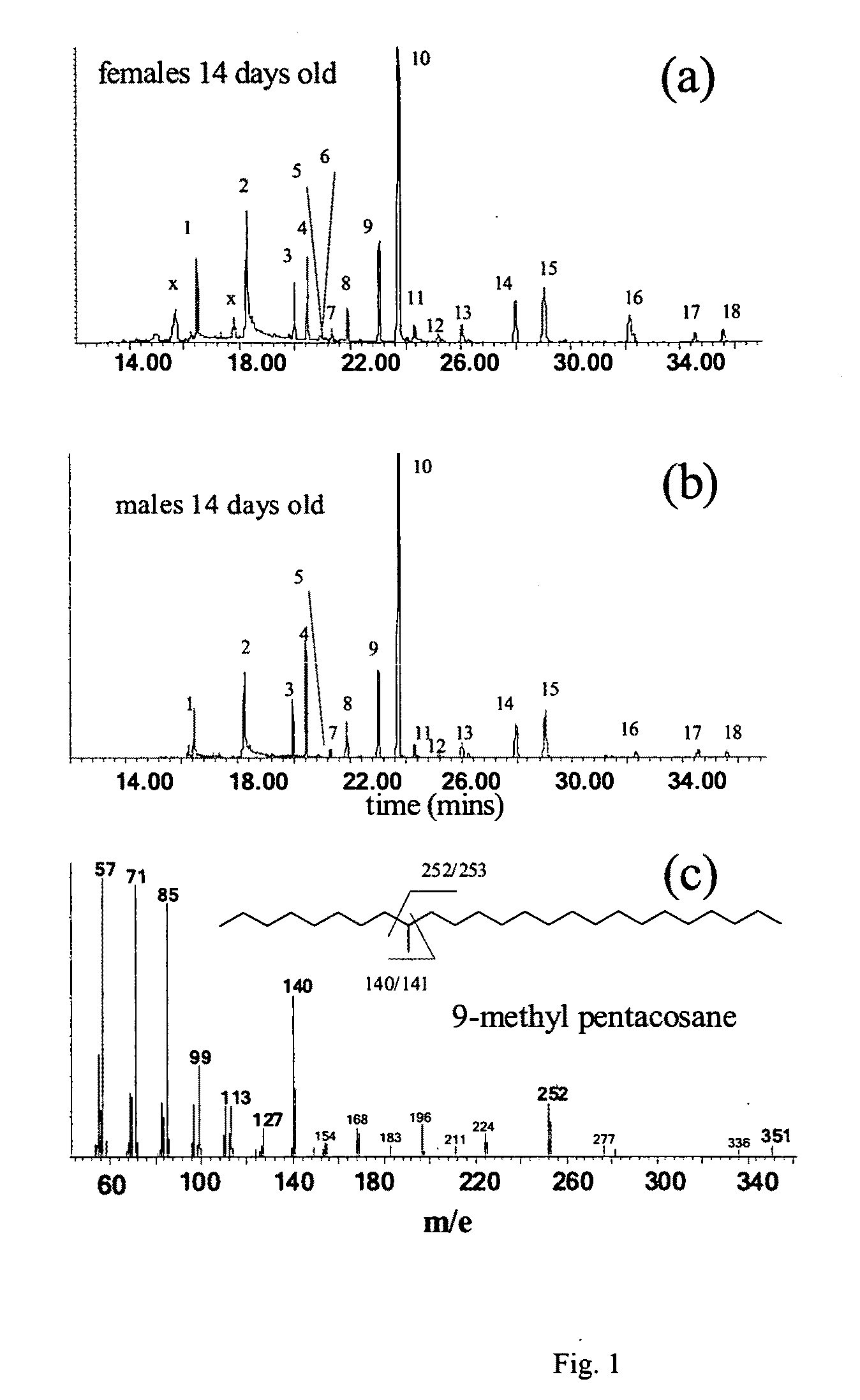
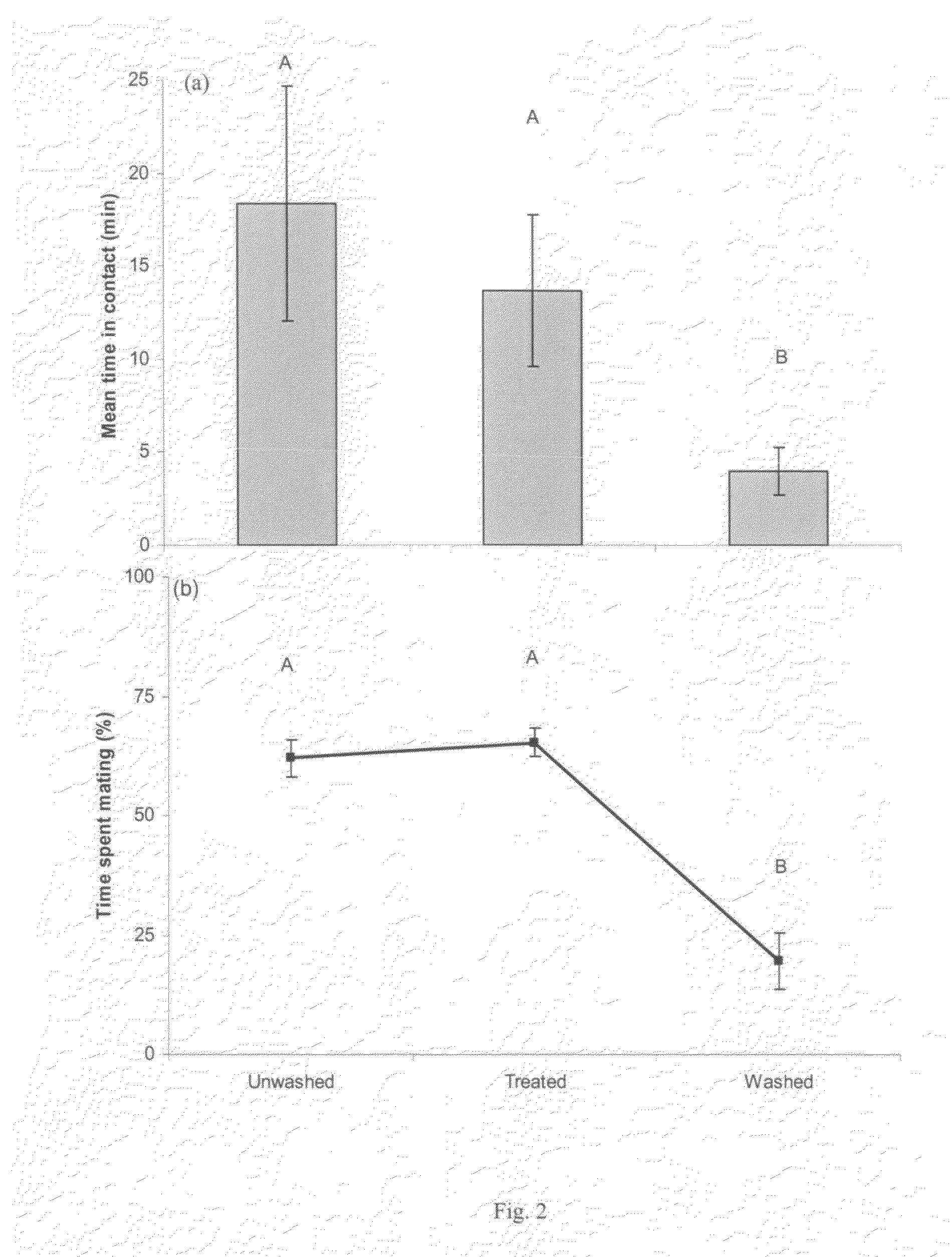
The invention disclosed relates to the detection of the Emerald Ash Borer (EAB), Agrilus planipennis Fairmaire (Coleoptra: B-prestidae) and in particular to the use of contact sex phenomones therefor. Analyses of the elytral hydrocarbons from male and female emerald ash borer, that were freshly emerged vs. sexually mature (>10 days old) revealed a female-specific compound, 9-methyl-pentacosane (9-Me-C25), only present in sexually mature females. This material was synthesized by the Wittig reaction of 2-decanone with (n-hexadecyl)-triphenylphosphonium bromide followed by catalytic reduction to yield racemic 9-Me C25, which matched the natural compound by GC / MS (retention time and EI-mass spectrum). In field bioassays with freeze-killed sexually mature A. planipennis females, feral males spent significantly more time in contact and attempting copulation with unwashed females than with females that had been washed in n-hexane to remove the cuticular hydrocarbons. Hexane-washed females to which 9-Me-C25 had been reapplied elicited similar contact time and percentage of time attempting copulation as unwashed females, indicating that 9-methyl-pentacosane is a contact sex-pheromone component of A. planipennis. This is the first contact sex pheromone identified in the Buprestidae.
Owner:NAT RES COUNCIL OF CANADA
Popular searches
Dispersed particle separation Ozone preparation Water/sewage treatment by oxidation Fatty acid oxidation Treatment with hydrotreatment processes Functional group formation/introduction Preparation by oxygen reduction Treatment with plural serial cracking stages only Organic oxidation Treatment with plural serial stages only
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com