Method for preparing 1,6-adipaldehyde
A technology of adipaldehyde and cyclohexene, which is applied in the field of fine chemical synthesis, can solve the problems of harsh low-temperature reaction conditions, expensive catalysts, and long reaction time, and achieve the effects of simple process, good industrial application prospects, and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
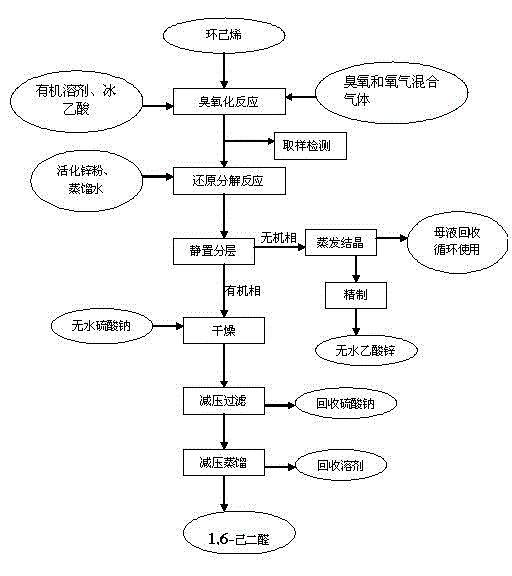
[0023] (1) Add 50mmol of cyclohexene, 15mL of dichloromethane and 6g (100mmol) of glacial acetic acid in a three-necked flask, put it in a low-temperature reaction bath, stir until the temperature of the system drops to -20℃, and pass O 3 / O 2 Carry out ozonation reaction. The tail gas first absorbs unreacted ozone through potassium iodide solution, and then recovers a small amount of volatile cyclohexene and organic solvent through glacial acetic acid. With 5% Br 2 / CCl 4 The solution detects the end of the reaction, after the oxidation is completed, pass 2 min N 2 Excess ozone is eliminated to obtain the ozonation reaction liquid, and the ozone compound directly enters the reduction reaction without separation.
[0024] (2) Transfer the ozonation reaction solution to a constant pressure dropping funnel, add it dropwise to 50mmol of activated zinc powder in distilled water, and complete the dropwise addition within 0.5h; stir for 50min at room temperature under nitrogen protectio...
Embodiment 2
[0026] (1) Add 50 mmol of cyclohexene, 25 mL of chloroform and 5.4 g (90 mmol) of glacial acetic acid in a three-necked flask, put it in a low-temperature reaction bath, stir until the temperature of the system drops to -10°C, and pass O 3 / O 2 Carry out oxidation reaction. The tail gas first absorbs unreacted ozone through potassium iodide solution, and then recovers a small amount of volatile cyclohexene and organic solvent through glacial acetic acid. With 5% Br 2 / CCl 4 The solution detects the end of the reaction, after the oxidation is completed, pass 2 min N 2 Excess ozone is eliminated to obtain the ozonation reaction liquid, and the ozone compound directly enters the reduction reaction without separation.
[0027] (2) Transfer the ozonation reaction solution to a constant pressure dropping funnel, add dropwise to 45mmol of distilled water containing activated zinc powder, and complete the dropwise addition within 0.5h; stir and react at room temperature under nitrogen pro...
Embodiment 3
[0029] (1) Add 50 mmol of cyclohexene, 40 mL of 1,2-dichloroethane and 6.6 g (110 mmol) of glacial acetic acid in a three-necked flask, put it in a low-temperature reaction bath, and stir until the temperature of the system drops to 5°C. O 3 / O 2 Carry out oxidation reaction. The tail gas first absorbs unreacted ozone through potassium iodide solution, and then recovers a small amount of volatile cyclohexene and organic solvent through glacial acetic acid. With 5% Br 2 / CCl 4 The solution detects the end of the reaction, after the oxidation is completed, pass 2 min N 2 Excess ozone is eliminated to obtain the ozonation reaction liquid, and the ozone compound directly enters the reduction reaction without separation.
[0030] (2) Transfer the ozonation reaction solution to a constant pressure dropping funnel, add it dropwise to 60mmol of distilled water containing activated zinc powder, and complete the dropwise addition within 0.5h; stir and react at room temperature for 75min un...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com