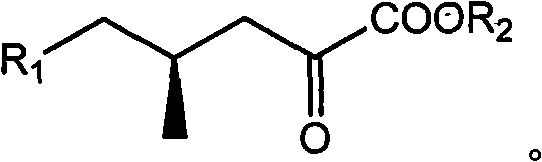
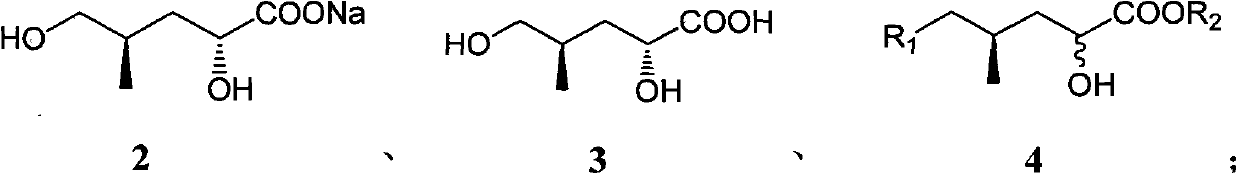
(4R)-4-methyl-2-carbonyl valerate compound, synthesizing method and application
A technology of carbonyl valerate and synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylate, etc., can solve problems such as single structure, and achieve the effect of single structure and stable compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The synthesis of embodiment 1 compound 3
[0029]
[0030] Take 2500g of crude compound (containing 20-30% of compound 2, if it contains more water, add toluene to reflux and divide the water to remove most of the water), under mechanical stirring, add anhydrous methanol to completely dissolve it, and slowly add concentrated hydrochloric acid Acidify to acidic pH=2, remove the white solid by suction filtration, spin dry the filtrate, add an appropriate amount of ethyl acetate to dissolve, dry with anhydrous sodium sulfate, filter, and spin off the solvent under reduced pressure to obtain 190 g of black oily liquid compound 3. The weight yield was 38%.
Embodiment 2
[0031] The synthesis of embodiment 2 compound 4a
[0032]
[0033] Take 100g of compound 3, add 500ml of toluene and 81g (3eq) of ground sodium hydroxide, reflux and divide water for 3 hours to remove the water absorbed during the grinding process of sodium hydroxide, slowly add 128ml (1.6eq) of benzyl bromide in portions ), reflux and divide water for 7 hours, add water after cooling to completely dissolve the system, extract twice with ether to remove impurities, add concentrated sulfuric acid to acidify to pH=1 under mechanical stirring, extract three times with ethyl acetate, combine the organic phases with saturated salt Wash once with water, dry with anhydrous sodium sulfate, filter, spin off the solvent under reduced pressure, dissolve in 600ml of anhydrous methanol, add 5ml (0.13eq) of concentrated sulfuric acid, stir at room temperature for 11 hours, dilute with water, spin off the methanol under reduced pressure, the water phase Extracted 3 times with ethyl acetat...
Embodiment 3
[0035] The synthesis of embodiment 3 compound 4b
[0036]
[0037]Take 100g of compound 3, add 500ml of toluene and 151g (4eq) of ground potassium hydroxide, reflux and divide water for 4 hours to remove the water absorbed during the grinding process of sodium hydroxide, slowly add 274ml of p-methoxybenzyl chloride (3eq), react at 80°C for 10 hours, add water after cooling to completely dissolve the system, extract twice with ether to remove impurities, add concentrated sulfuric acid to acidify to pH=2 under mechanical stirring, extract three times with ethyl acetate, and combine the organic phases Wash once with saturated brine, dry over anhydrous sodium sulfate, filter, spin off the solvent under reduced pressure, dissolve in 600ml of anhydrous methanol, add 3.6ml of concentrated sulfuric acid (0.1eq), stir at room temperature for 10 hours, dilute with water, spin off under reduced pressure Methanol, the aqueous phase was extracted 3 times with ethyl acetate, the organic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com