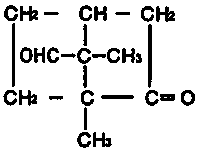
Separation and purification method of oxidized camphor
A technology of separation and purification and camphor, which is applied in the field of chemical synthesis, can solve the problems that crystallization post-treatment methods cannot be removed, cannot meet the needs of separation, and the conditions of impurity synthesis are uncontrollable, etc., to solve the problems of insufficient selectivity, large sample loading, and easy The effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1.5 g of crude oxidized camphor was dissolved in 7.5 mL of n-hexane-ethyl acetate, and the injection volume was 7.5 mL; a carboxyl silica gel column (column size 50×250 mm, particle size 10 microns, pore size 10 nm, packing mass 300 g) was used , column temperature 30 o C, flow rate 80mL / min; non-polar solvent is n-hexane, polar solvent is ethyl acetate, wherein the volume ratio of non-polar solvent / polar solvent is 90 / 10; ultraviolet detector, detection wavelength 280 nm, from the target Pick up fractions from the peak when the peak comes out and stop when the peak returns to the baseline. During the process of picking up fractions, pick up fractions every 3 minutes, and combine the fractions based on the results of liquid phase fraction analysis. After purification, the purity can be more than 98%, and the purity is less than 1%.
Embodiment 2
[0023] 0.3 g of crude oxidized camphor was dissolved in 2 mL of n-hexane-ethyl acetate solution, and the injection volume was 2 mL; a carboxyl silica gel column (column size 50×250 mm, particle size 10 microns, pore size 30 nm, packing mass 300 g ), column temperature 30 o C, flow rate 80 mL / min; non-polar solvent is n-hexane, polar solvent is ethyl acetate, wherein the volume ratio of non-polar solvent / polar solvent is 90 / 10; ultraviolet detector, detection wavelength 280 nm, from When the target peak comes out of the peak, pick up fractions until the peak returns to the baseline and stop. During the fraction pick-up process, pick up fractions every 3 minutes, and combine the fractions through the analysis results of liquid phase fractions. After purification, the purity can be more than 98%, and the purity is less than 1%. .
Embodiment 3
[0025] 10 g of crude oxidized camphor was dissolved in 50 mL of n-hexane-ethyl acetate solution, and the injection volume was 50 mL. A carboxyl silica gel column (column size 100×250 mm, particle size 10 microns, pore size 20 nm, packing mass 1.2 kg) was used, and the column temperature was 30 o C, flow rate 240 mL / min. The non-polar solvent is n-hexane, and the polar solvent is ethyl acetate, wherein the volume ratio of non-polar solvent / polar solvent is 90 / 10; the ultraviolet detector, the detection wavelength is 280 nm, and the fraction is connected from the target peak to the peak. The peak returns to the baseline and stops. During the fraction collection process, the fractions are collected every 3 minutes, and the fractions are combined through the analysis results of the liquid phase fractions. After purification, the purity can be more than 98%, and the purity is less than 1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com