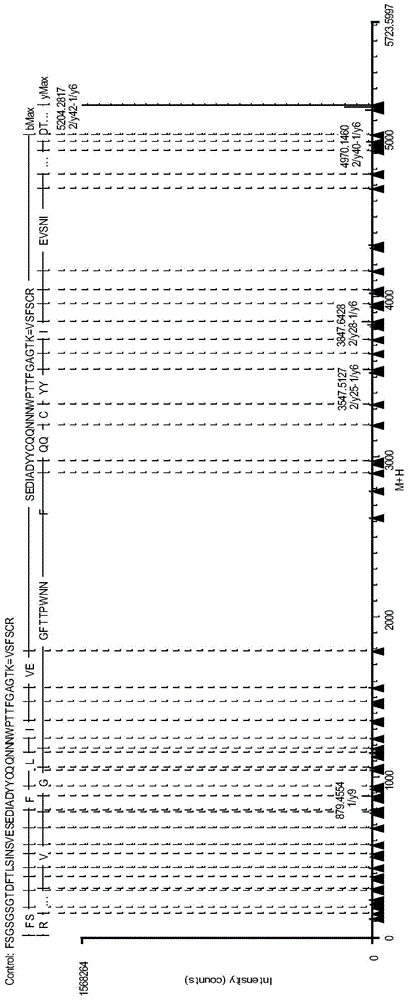
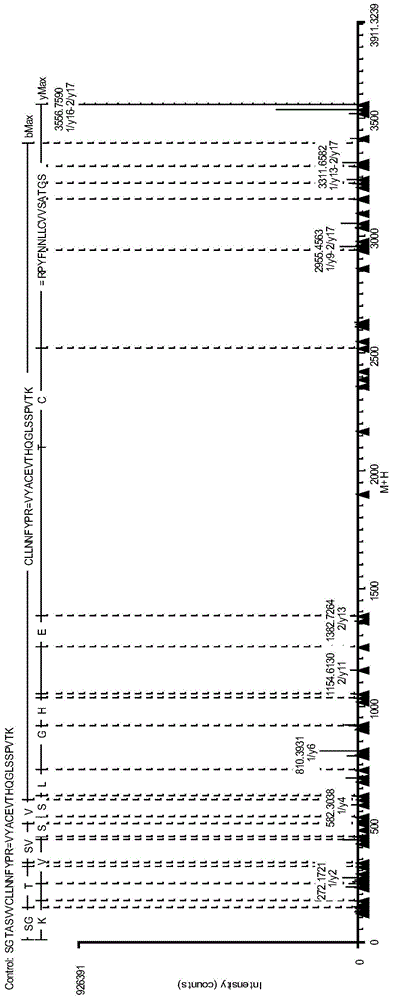
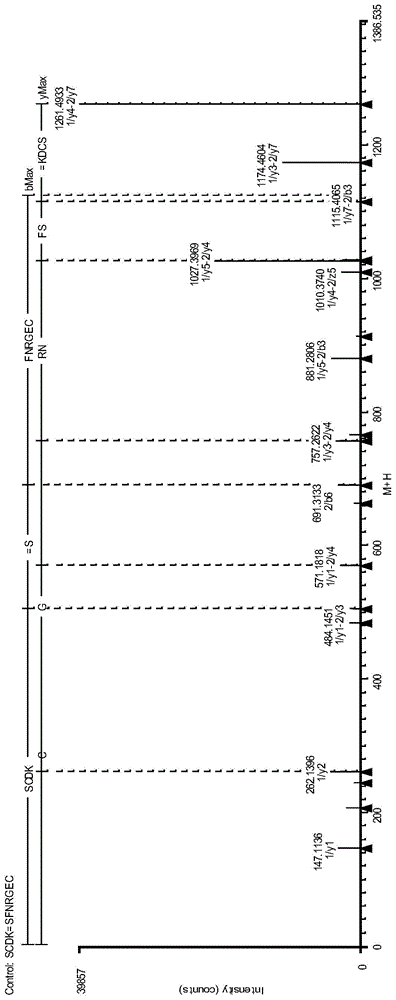
Antibody protein disulfide bond paired analysis method
An analysis method, disulfide bond technology, applied in the field of antibody disulfide bond pairing analysis, to achieve the effect of rich fragment ions and less enzyme cleavage methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Analysis of antibody protein disulfide bond pairing (trypsin combined with Lys-C digestion)
[0047] Antibody protein disulfide bond pairing analysis steps:
[0048] (1) Removal of N-Glycosylations
[0049] Take 1mg antibody protein sample, add 50mM NH 4 HCO 3 (pH 8.0) solution to a volume of 20-50 μL, add 1 μL of peptide N-glycosidase PNGase F (NEB, 500000u / mL), mix well, and incubate at 37°C for 24 hours.
[0050] (2) Protein denaturation treatment
[0051] Take 0.5 mg protein sample and add it to 380 μl denaturation buffer (8M guanidine hydrochloride+5mM EDTA+0.5MTris, pH8.3), mix well, and incubate at 37°C for 60 minutes. Ultrafiltration centrifugation or desalting column desalting to 50mM NH 4 HCO 3 (pH8.0) buffer.
[0052] (3) Enzymolysis
[0053] Take 100 μg of the protein in (2), add Trypsin at 1:20 (wt / wt), add Lys-C at 1:200, combine with enzymatic hydrolysis of antibody protein, and incubate at 37°C for 4 hours.
[0054] (4) Reduction and ...
Embodiment 2
[0070] Example 2: Analysis of the complete molecular weight of antibody protein (trypsin digestion)
[0071] Antibody protein disulfide bond pairing analysis steps:
[0072] (1) Removal of N-Glycosylations
[0073] Take 1mg antibody protein sample, add 50mM NH 4 HCO 3 (pH 8.0) solution to a volume of 20-50 μL, add 1 μL of peptide N-glycosidase PNGase F (NEB, 500000u / mL), mix well, and incubate at 37°C for 24 hours.
[0074] (2) Protein denaturation treatment
[0075] Take 0.5 mg protein sample and add it to 380 μl denaturation buffer (8M guanidine hydrochloride+5mM EDTA+0.5MTris, pH8.3), mix well, and incubate at 37°C for 60 minutes. Ultrafiltration centrifugation or desalting column desalting to 50mM NH 4 HCO 3 (pH8.0) buffer.
[0076] (3) Enzymolysis
[0077] Take 100 μg of the protein in (2), add Trypsin to digest the antibody protein at 1:20 (wt / wt), and incubate at 37°C for 4 hours.
[0078] (4) Reduction and termination of enzymatic hydrolysis
[0079] After th...
Embodiment 3
[0084] Example 3: Analysis of the complete molecular weight of antibody protein (Lys-C digestion)
[0085] Antibody protein disulfide bond pairing analysis steps:
[0086] (1) Removal of N-Glycosylations
[0087] Take 1mg antibody protein sample, add 50mM NH 4 HCO 3 (pH 8.0) solution to a volume of 20-50 μL, add 1 μL of peptide N-glycosidase PNGase F (NEB, 500000u / mL), mix well, and incubate at 37°C for 24 hours.
[0088] (2) Protein denaturation treatment
[0089] Take 0.5 mg protein sample and add it to 380 μl denaturation buffer (8M guanidine hydrochloride+5mM EDTA+0.5MTris, pH8.3), mix well, and incubate at 37°C for 60 minutes. Ultrafiltration centrifugation or desalting column desalting to 50mM NH 4 HCO 3 (pH8.0) buffer.
[0090] (3) Enzymolysis
[0091] Take 100 μg of the protein in (2), add Lys-C enzymatic antibody protein at a ratio of 1:200, and incubate at 37°C for 4 hours.
[0092] (4) Reduction and termination of enzymatic hydrolysis
[0093] After the enzy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com