Effect of lipophilic nutrients on diabetic eye diseases
A diabetic and diabetic technology, applied in the field of the effect of lipophilic nutrients on diabetic eye disease, can solve the problem of difficult delivery of therapeutic/preventive drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0145] In some embodiments, a method for preparing a novel water-soluble composition with enhanced bioavailability comprises:
[0146] (i) dissolving curcumin, at least one antioxidant, hydrophilic carrier and fat in a solvent to form a homogeneous mixture;
[0147] (ii) heating the obtained mixture at a temperature range of 25°C-60°C for 4-8 hours to obtain a dry-wet mixture;
[0148] (iii) removing the solvent by evaporation to form a dry mixture; and
[0149] (iv) Grinding the dry blend to obtain a fine powder.
[0150] The curcumin used in step (i) can be commercially available, and its content range is 85-96%. It can also be curcumin-rich turmeric extract. The amount of curcumin added may be sufficient to make the water-soluble curcumin contain 1-55% curcumin.
[0151] The antioxidant used in step (i) may be selected from: natural tocopherol, ascorbyl palmitate, rosemary extract, epigallocatechin, catechin, ascorbic acid and mixtures thereof. The amount of antioxidan...
Embodiment 1
[0163] UltraSol Lutemax2020 TM and UltraSol CurcuWin TM Effect on diabetic cataract
[0164] experimental design
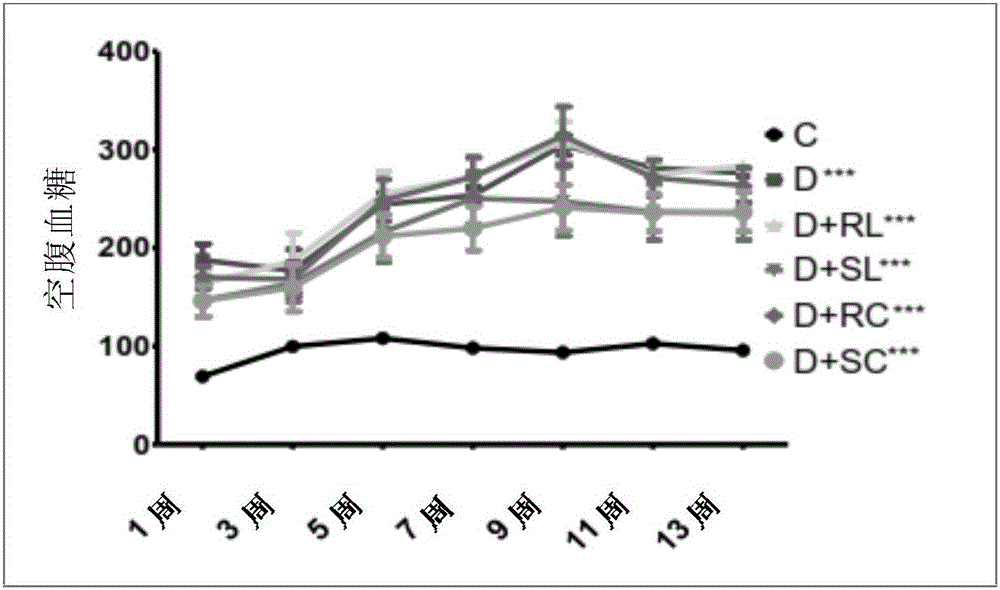
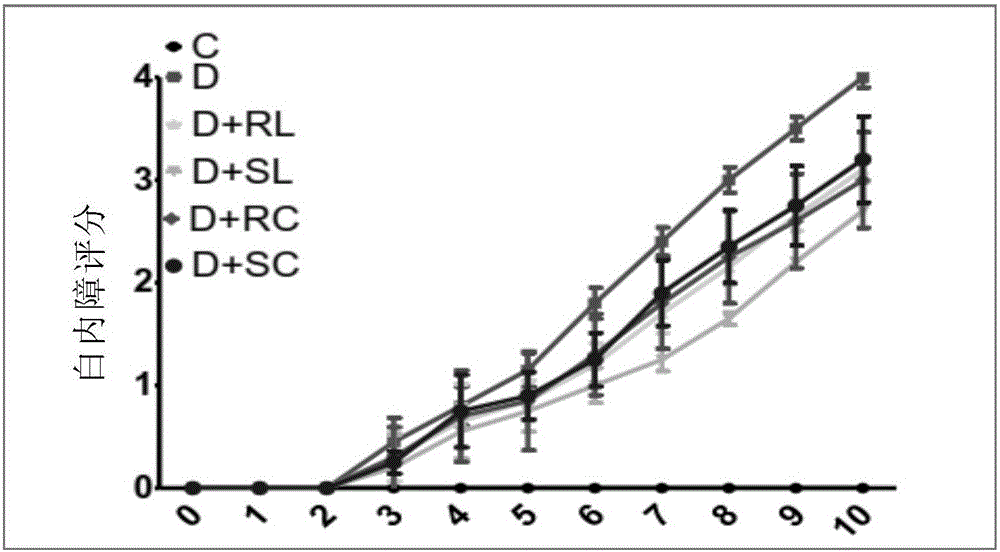
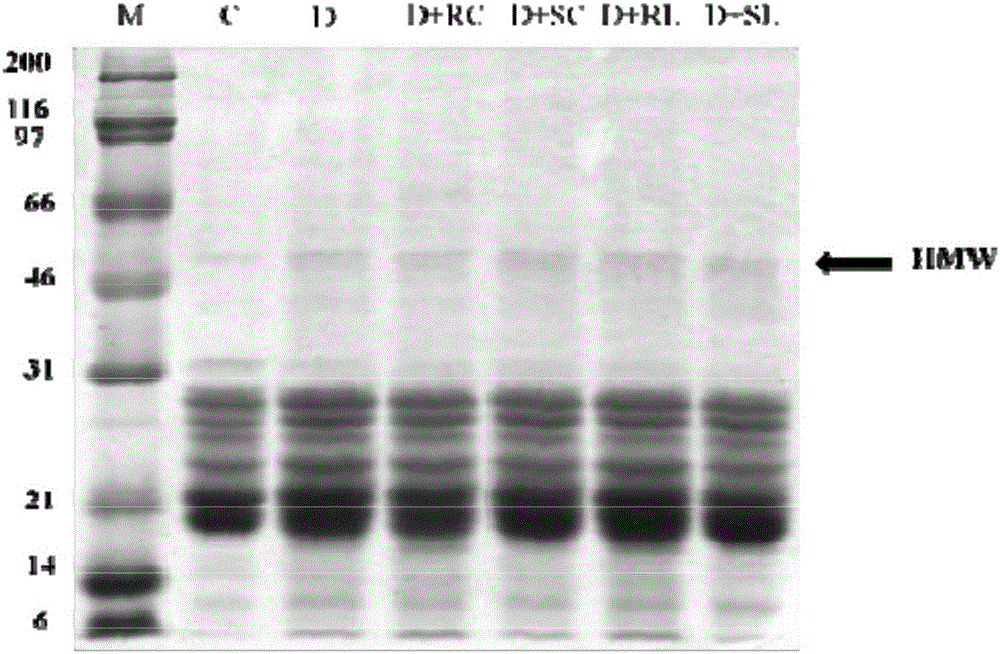
[0165]Male Wistar strain (WNIN) rats (2 months old; mean BW 213±14 g) were obtained from the National Center for Laboratory Animal Science, National Institute of Nutrition, Hyderabad, India. Animals were maintained on NCLAS, NIN, and acclimatized in the laboratory for 2 weeks. Overnight fasted animals were injected intraperitoneally with a single dose of STZ (30 mg / kg) in 0.1 M citrate buffer, pH 4.5. Another group of rats received vehicle only as a control (Group I; n=12). Fasting blood glucose levels were measured 72 hours after STZ injection. Animals with blood glucose levels >150 mg / dL were considered diabetic and they were divided into 5 groups (Groups II-VI). A control group of rats (n=6) was fed with 0.01% soluble curcumin (group VII) and 0.5% soluble lutein (group VIII) alone.
[0166] All animals were housed individually and maintained on their res...
Embodiment 2
[0206] UltraSol Lutemax2020 TM -Impact on diabetic retinopathy (by genetic nutrition approach)
[0207] Determination of soluble lutein by genetic nutrition method (UltraSol Lutemax2020 TM ) beneficial effect on the retina of diabetic rats and compared this effect with common lutein
[0208] Methodology
[0209] Animal model: Streptozotocin (STZ) diabetic rat model is one of the most commonly used models of human diabetes. It is known to mimic a variety of acute and some chronic complications observed in human diabetes. The strength of this model is that it is highly reproducible, and the timelines for the development of various complications are well established and reproducible. Given the similarity of certain structural, functional, and biochemical abnormalities in the human disease, it is considered an appropriate model to evaluate the mechanisms of diabetes and its potential treatments.
[0210] Experimental design: Male Wistar-NIN (WNIN) rats (2 months old; mean B...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com