Construction method and application of xenograft tumor models derived from different subtypes of Chinese breast cancer patients
A construction method and xenotransplantation technology, which are applied in the direction of medical raw materials derived from mammals, medical preparations containing active ingredients, and pharmaceutical formulas, can solve the problems of unrecorded success rate of primary PDX, irreversible changes in natural attributes, loss of Issues such as the characteristics of primary tumors, to achieve the effect of increasing the success rate of construction, stability and lasting stimulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: Construction method described in the present invention (construct primary human breast cancer xenograft tumor model)
[0045] Take human breast cancer tissue specimens, put them into a sterile 4°C pre-cooled 15ml centrifuge tube filled with DMEM basal medium, keep them at 0-4°C, and process them within 1 hour.
[0046] In the ultra-clean workbench, put the above tumor tissue into a sterile petri dish, cut off the surrounding adipose tissue and necrotic tissue, and use a sterile scalpel to cut the tumor tissue P 0 Cut 3 After the small piece was removed, transfer it into a sterile 1.5ml EP tube, add 200ul each of calf serum and matrigel (BD company, product number 354234), and use a 1ml syringe to change 12 # Aspirate tumor tissue with a needle for use;
[0047] The 18-22g, 6-8 week old SPF grade NOD-SCID female mice were used for inoculation. The specific vaccination method: after the mice were anesthetized, the abdomen and the back of the neck of the mic...
Embodiment 2
[0048] Example 2: Primary transplanted tumor model breast cancer transplanted tumor passage
[0049] Waiting for Example 1 Primary tumor-bearing mouse tumor grows to 300-500mm 3 The tumor-bearing mice were selected to be sacrificed by carbon dioxide asphyxiation, and the body surface was sterilized. The breast cancer xenograft tumor tissue in the primary mouse model was dissected under a sterile environment, chopped and then digested with digestive juice (collagenase (Stemcell Co., Ltd.). , Cat. No. 07912) 1 part: M199 medium (Corning Company, Cat. No. R10-060-cv) 9 parts), digested in a water bath at 37°C for 1 hour, filtered to obtain a single-cell suspension, centrifuged at 350×g for 5 minutes to collect cells, and used After resuspending the cells with calf serum, count the number of living cells with trypan blue and adjust the cell concentration to 1×10 7 / ml, using a 1ml syringe to implant into the fourth pair of breast pads of SPF grade NOD-SCID female mice at the age ...
Embodiment 3
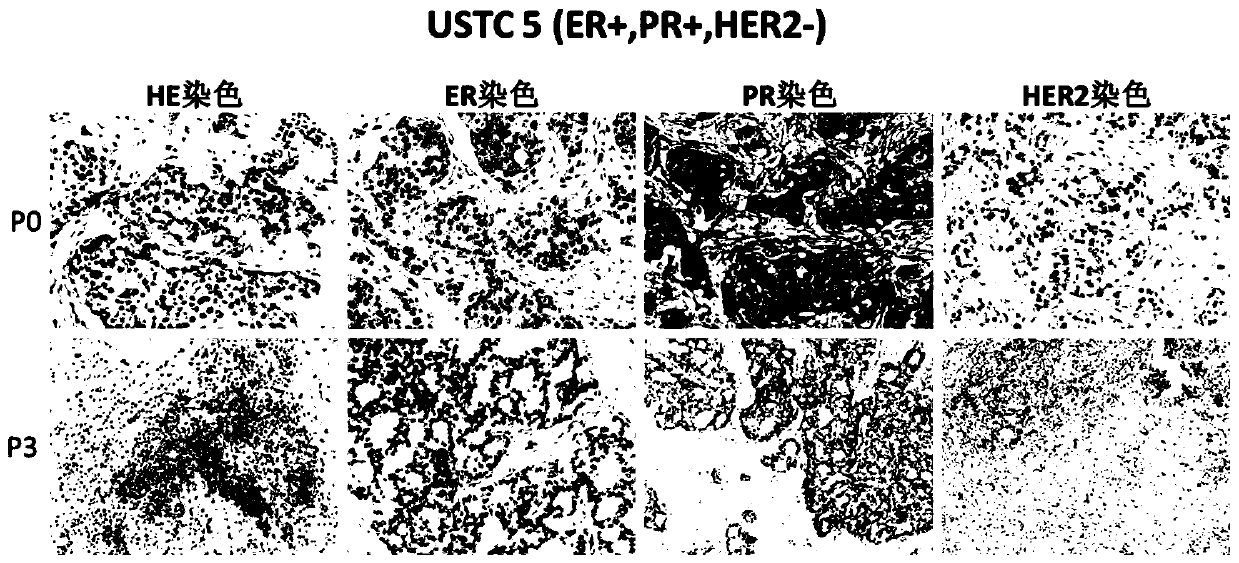
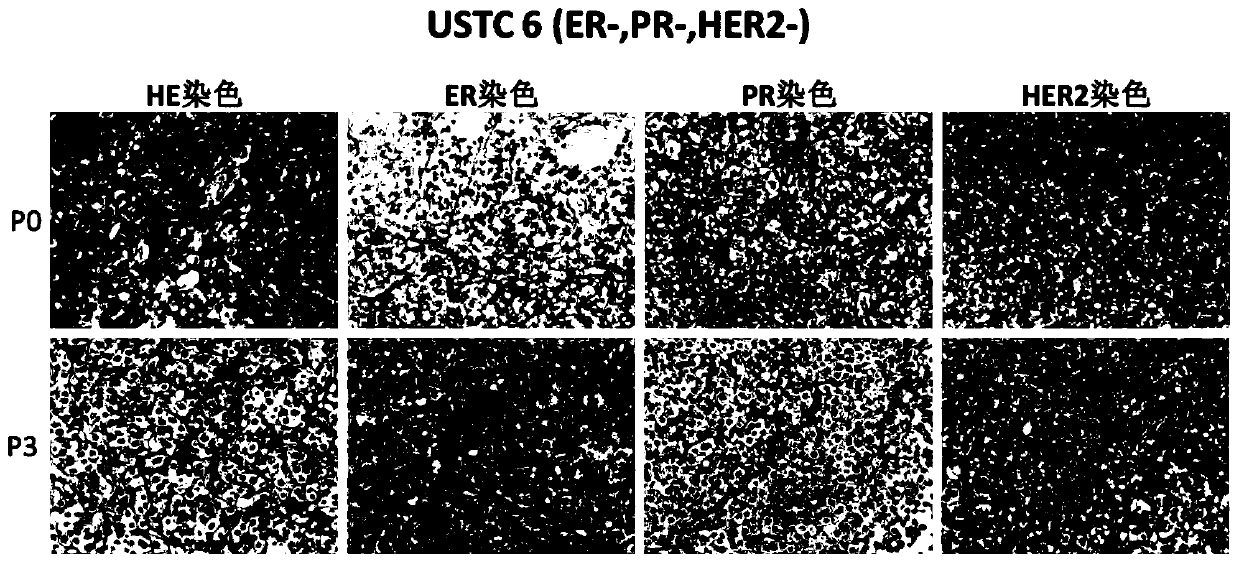
[0050] Example 3: Stable passage test of breast cancer tissues of different subtypes
[0051] The test was carried out with 12 cases of clinical human breast cancer tissues as specimens according to the construction method of Example 1 and the passaging method of Example 2 of the present invention, and the basic characteristics of breast cancer tissues between generations were counted, and the results are shown in Table 1.
[0052] Table 1 Stable passage test of different subtypes of breast cancer tissues
[0053]
[0054] Note: The number of passages listed in the table "Maintaining Stable Passage of Molecular Typing Markers" does not mean that it can only be passed to the listed number of passages, and it can be passed stably all the time. Passage data cannot be infinitely recorded in the table.
[0055] It can be seen from Table 1 that the successful primary xenograft tumor model constructed according to the construction method of the present invention can be stably pas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com