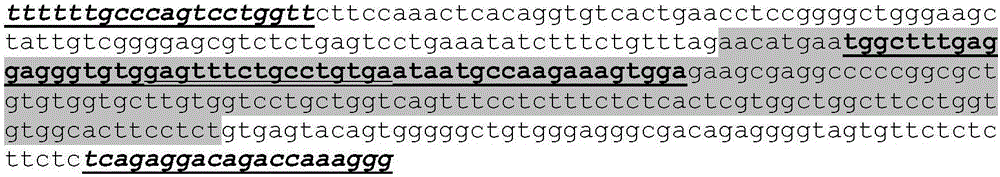Novel vertebrate cells and methods for recombinantly expressing a polypeptide of interest
A vertebrate cell and cell technology, applied in the field of new vertebrate cells and methods for recombinant expression of polypeptides of interest, can solve problems such as expensive and time-consuming adaptation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0101] According to one embodiment, the method comprises
[0102] (a) cultivating the vertebrate host cell described in the first aspect under certain conditions that allow expression and secretion of the polypeptide of interest;
[0103] (b) isolating the polypeptide of interest from the cell culture medium; and
[0104] (c) Optional processing of the isolated polypeptide of interest.
[0105] Therefore, as a preferred embodiment of the method described in the third aspect, a method for recombinantly producing a polypeptide of interest is provided, comprising
[0106] (a) cultivating the vertebrate host cell described in the first aspect under conditions that allow expression and secretion of the polypeptide of interest into the cell culture medium;
[0107] (b) isolating the polypeptide of interest from the cell culture medium; and
[0108] (c) Optional processing of the isolated polypeptide of interest.
[0109] The host cells can be cultured under serum-free conditions...
Embodiment 1
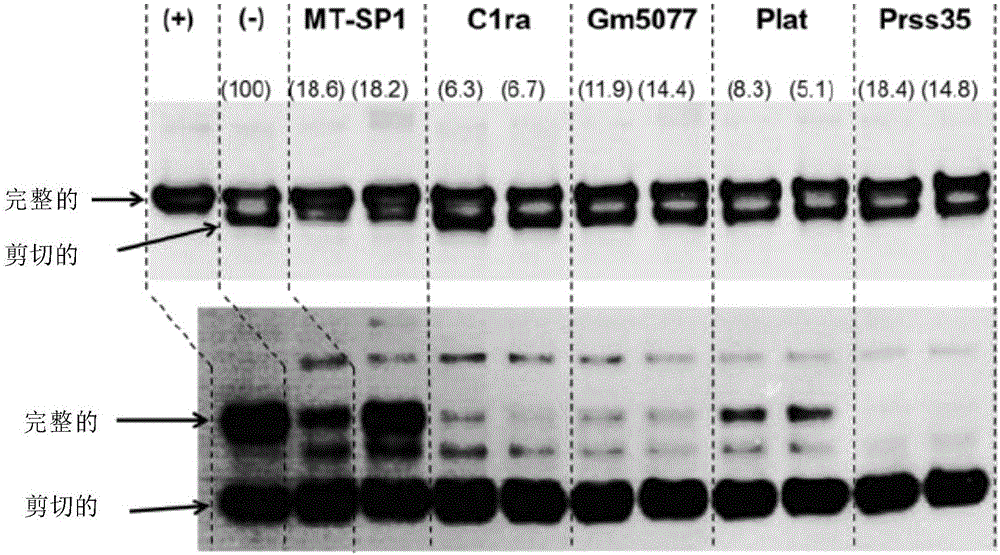
[0167] II. Example 1: Cleavage of different polypeptides of interest when expression of different proteases is silenced by RNA interference (RNAi)
[0168] First, the proteolytic cleavage sites of different recombinant proteins prone to cleavage were analyzed by different techniques, including CE-SDS analysis, mass spectrometry and computer simulation analysis (data not shown). This analysis, in conjunction with other assays employing different protease inhibitors, revealed that the majority of the tested proteins tended to be cleaved by trypsin-like proteases, a subfamily of serine proteases. The RNAi experiments of Example 1 were set up to demonstrate that interstitial protease is the key protease responsible for cleavage and that silencing of the interstitial protease gene can significantly reduce cleavage, whereas silencing of other genes encoding different proteases does not reduce cleavage of the polypeptide of interest. siRNAs were designed against the following target mR...
Embodiment 2
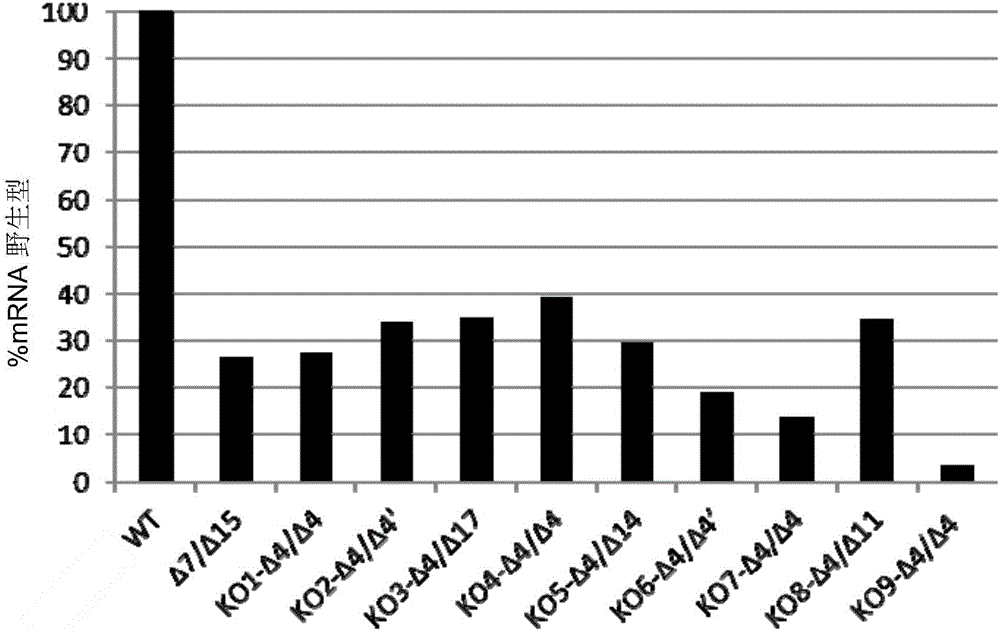
[0190] III. Example 2: Interstitial protease gene knockout (KO) in CHO cells results in reduced cleavage
[0191] A. KO with TALEN technology
[0192] Nine interstitial protease (MT-SP1) knockout cell clones were generated based on CHO-K1 derived cells, using TALEN (transcription activator-like effector nuclease) technology. For the knockout, exon 2 of the interstitial protease was targeted to the region preceding the coding region of the transmembrane domain. Exon 2 was chosen because it covers different alternative splicing variants. Incorporating a frameshift mutation in exon 2 has the advantage that the truncated protein is shorter and intracellular, and moreover, is unstable and nontoxic to cells.
[0193] 2.A.1. Design / preparation and application of interstitial protease exon 2 specific TALEN
[0194] Sequencing of interstitial protease exon 2 and flanking introns in a CHO-K1-derived parental cell line (see figure 2 , SEQ ID NO: 31).
[0195] Design of 2 truncated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com