Novel coronavirus S protein and subunit vaccine thereof
A subunit vaccine, coronavirus technology, applied in the field of new coronavirus S protein and its subunit vaccine, can solve the problems of unknown integration risk, interference with S protein immunogenicity, inability to effectively deal with mutant strains, etc. cut effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0069] According to another typical implementation of the embodiments of the present invention, a method for preparing the S protein of a novel coronavirus is provided, the method comprising:
[0070] obtaining the recombinant expression vector;
[0071] Transfecting the recombinant expression vector into cells, and obtaining a cell line stably expressing the recombinant S protein through glutamine resistance screening and monoclonal screening of the cell population;
[0072] The cell line was secreted, expressed and purified to obtain a purified recombinant novel coronavirus S protein.
[0073] According to another typical implementation of the embodiments of the present invention, a novel coronavirus subunit vaccine is provided, and the novel coronavirus subunit vaccine comprises the recombinant S protein and a pharmaceutically acceptable adjuvant.
[0074] The adjuvant includes at least one of aluminum hydroxide, lecithin, Freund's adjuvant, MPL TM, IL-12, aluminum hydroxi...
Embodiment 1
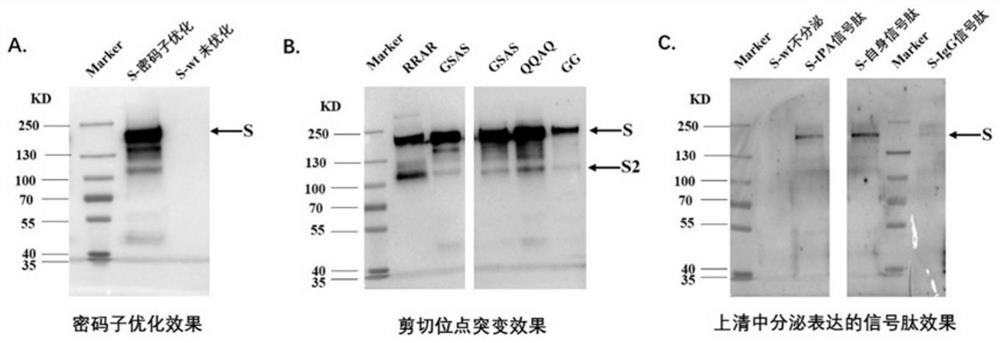
[0078] Example 1 Recombinant S protein vector construction and expression optimization
[0079] 1. Construction of S protein gene expressed in mammalian cell supernatant
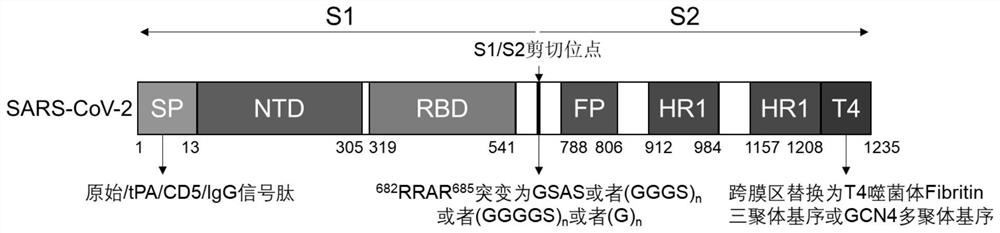
[0080] The schematic diagram of the construction of the S protein expression gene of the present invention is shown in figure 1 . figure 1 Sequence design diagram of mammalian cell secreted S protein: the sequence retains the original signal peptide, or is mutated to tPA signal peptide / CD5 signal peptide / IgG signal peptide; Furin cleavage site is mutated from RRAR to GSAS, GS combination, (GGGS) n or (GGGGS) n or (G) n ; The C-terminal transmembrane region and the intracellular region were replaced by the minor fibrin sequence of T4 phage.
[0081] First, in order to efficiently express in eukaryotic cells, we used the JAVA Codon Adaptation software to optimize the codons of the S expression gene with mammalian preference. The nucleotide sequence of the original signal peptide was optimized as SEQ ID N...
Embodiment 2
[0093]Embodiment 2. Recombinant S protein trimer vaccine immunization and effect identification
[0094] 1. Immunization procedure of mice
[0095] The mice used in this experiment were K18-hACE male mice, 6-8 weeks old, 19-28g, purchased from Jiangsu Jicui Yaokang Biotechnology Co., Ltd. All animal experiments were performed in SPF laboratory. The experimental group was inoculated with vaccine + adjuvant, a total of 4 mice; the control group was inoculated with PBS, a total of 4 mice. The second injection was inoculated 14 days after the first injection, and 35 days after the first injection (that is, 21 days after the second injection), blood was collected from the orbit of all mice. Mouse serum was taken for testing.
[0096] 2. ELISA detection process
[0097] (1) Dilute RBD (Shenzhou Science and Technology Co., Ltd., Yiqiao Shenzhou Technology Co., Ltd.) with 0.1M carbonate buffer (pH=9.6) to make the final concentration 1ng / μL, and then add 100μL to each well of the 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com