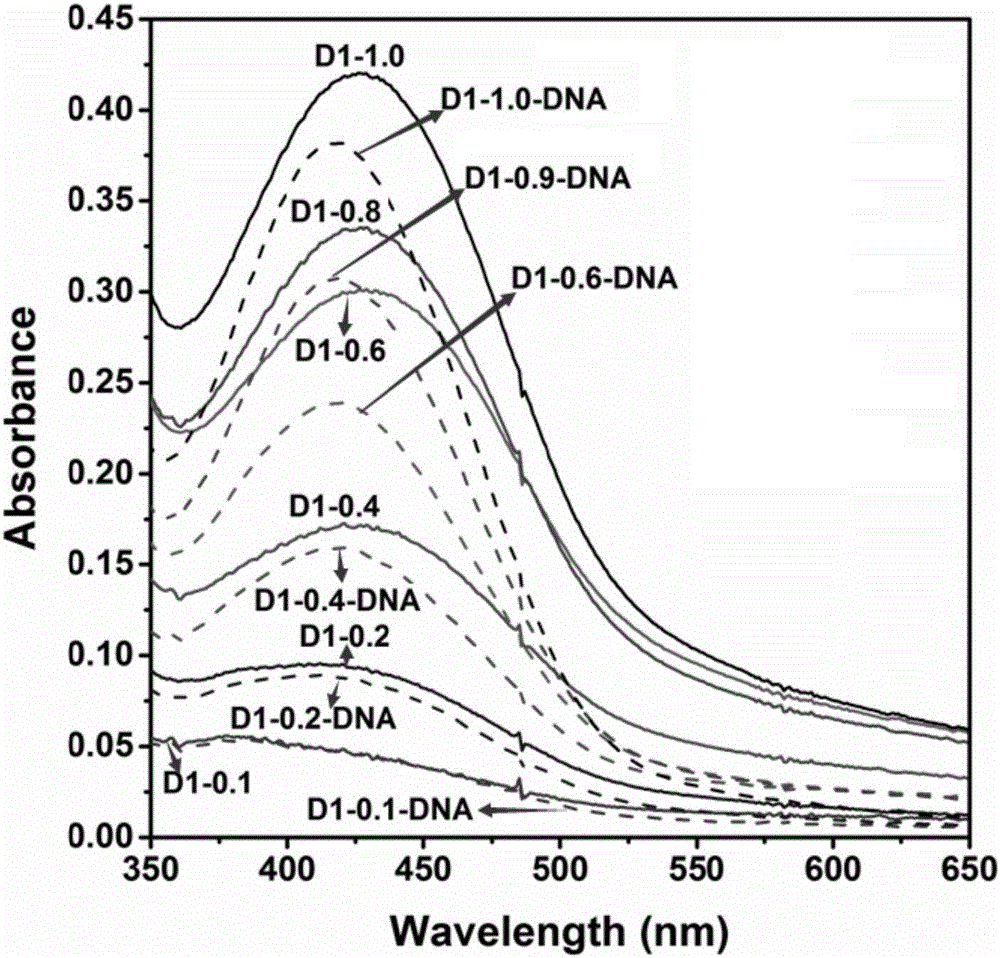
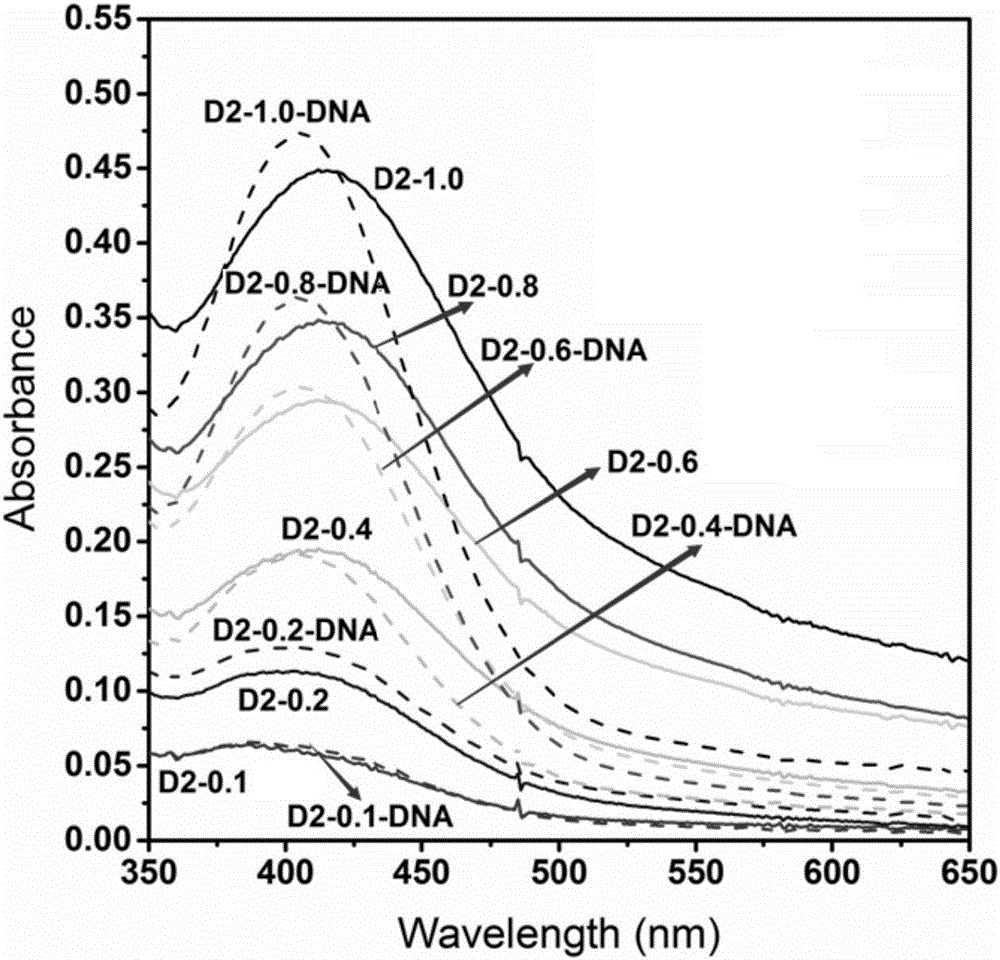
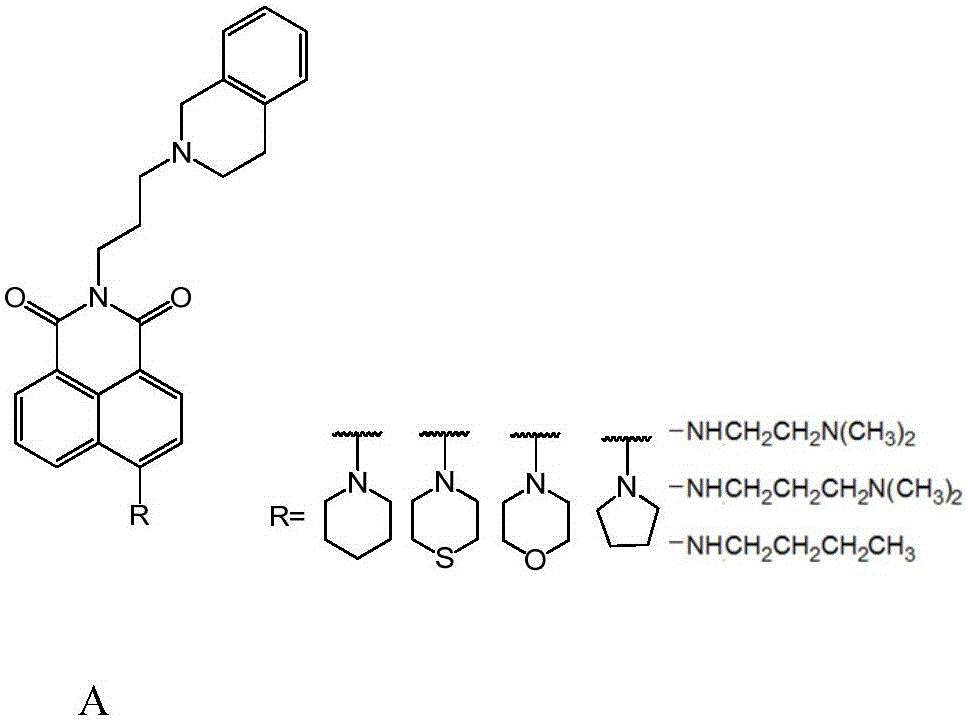
1,8-anhydride naphthalene derivative with side chain containing isoquinoline and synthesis and application thereof
A technology of isoquinoline and naphthalene anhydrides, which is applied in the field of bioorganic synthesis, can solve the problems of limited clinical application, high toxicity of arinafetide and mitonaftamide, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Synthesis of N-[3'-(3",4"-dihydroisoquinolin-2(1H)-yl)-propyl]-4-piperidinyl-1,8-naphthalene anhydride:
[0021] (1) Synthesis of intermediate 1 4-R base-1.8-naphthalene anhydride:
[0022]
[0023] Synthesis of intermediate 4-piperidinyl-1,8-naphthalene anhydride:
[0024] Take 2.77g (10mmol) of 4-bromo-1,8-naphthalene anhydride and place it in a 100mL double-neck round bottom flask, add 40mL of ethylene glycol monomethyl ether as a solvent, add 0.92mL (10mmol) of piperidine, and heat up to 125 °C, stirred and refluxed for 3 hours, cooled at room temperature for half an hour, poured into 200ml of water to precipitate precipitates, filtered with suction and dried to obtain 2.6g of the product with a yield of 92.53%.
[0025] (2) Synthesis of intermediate N-(3'-hydroxyl-propyl)-4-R base-1,8-naphthalene anhydride:
[0026]
[0027] Synthesis of intermediate N-(3'-hydroxy-propyl)-4-piperidinyl-1,8-naphthalene anhydride:
[0028] Take 2.5g (8.8mmol) of 4-piperidiny...
Embodiment 2
[0040] Synthesis of N-[3'-(3",4"-dihydroisoquinolin-2(1H)-yl)-propyl]-4-thiomorpholinyl-1,8-naphthalene anhydride:
[0041] (1) Synthesis of intermediate 4-thiomorpholino-1,8-naphthalene anhydride:
[0042] 1.01 mL (10 mmol) of thiomorpholine was added instead of piperidine, and the rest of the synthesis process was the same as in Example 1 to obtain 2.7 g of the product with a yield of 90.30%.
[0043] (2) Synthesis of intermediate N-(3'-hydroxy-propyl)-4-thiomorpholino-1,8-naphthalene anhydride:
[0044] Take 2.5g (8.4mmol) of 4-thiomorpholino-1,8-naphthalene anhydride instead of 4-piperidinyl-1,8-naphthalene anhydride, add 0.7mL (9.24mmol) of n-propanolamine, and the rest of the synthesis process Same as in Example 1, 2.5 g of product was obtained with a yield of 83.61%.
[0045] (3) Synthesis of intermediate N-(3'-bromo-propyl)-4-thiomorpholino-1,8-naphthalene anhydride:
[0046] Take 2.2g (6.2mmol) of N-(3'-hydroxy-propyl)-4-thiomorpholino-1,8-naphthalene anhydride inste...
Embodiment 3
[0054] Synthesis of N-[3'-(3",4"-dihydroisoquinolin-2(1H)-yl)-propyl]-4-morpholinyl-1,8-naphthalene anhydride:
[0055] (1) Synthesis of intermediate 4-morpholino-1,8-naphthalene anhydride:
[0056] 0.87mL (10mmol) morpholine was added instead of piperidine, and the rest of the synthesis process was the same as in Example 1 to obtain 2.5g of product with a yield of 88.34%.
[0057] (2) Synthesis of intermediate N-(3'-hydroxyl-propyl)-4-morpholinyl-1,8-naphthalene anhydride:
[0058] Take 2.4g (8.5mmol) 4-morpholino-1,8-naphthalene anhydride instead of 4-piperidinyl-1,8-naphthalene anhydride, add 0.71mL (9.35mmol) n-propanolamine, and carry out the rest of the synthesis process Example 1, 2.3g of product was obtained, yield 79.58%.
[0059] (3) Synthesis of intermediate N-(3'-bromo-propyl)-4-morpholinyl-1,8-naphthalene anhydride:
[0060] Take 2.2 g (6.5 mmol) of N-(3'-hydroxy-propyl)-4-morpholinyl-1,8-naphthalene anhydride instead of N-(3'-hydroxy-propyl)-4-piperidinyl- Fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com