Method for synthesizing and purifying Fasudil hydrochloride
A technology of fasudil hydrochloride and purification method, which is applied in the field of synthesis and purification of fasudil hydrochloride, can solve problems such as difficult removal, achieve the effects of reducing the generation of impurities, solving the problem of excessive residual impurities, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
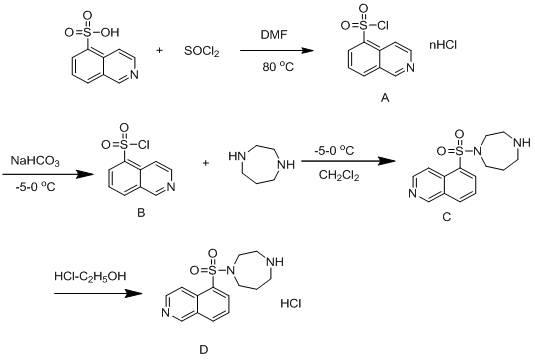
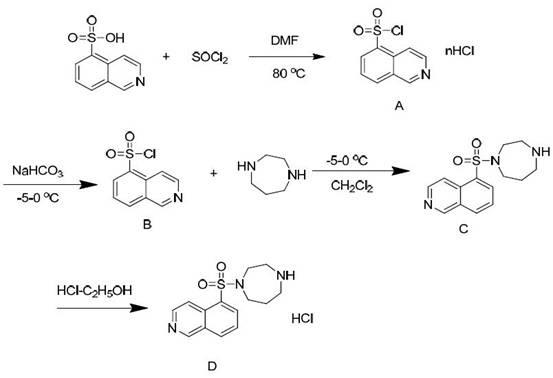
[0031] A method for synthesizing and purifying fasudil hydrochloride, using 5-isoquinolinesulfonic acid as a starting material, and obtaining a high-purity product through steps including chlorination, synthesis with homopiperazine, and acid-base treatment, is characterized in that it includes Follow the steps below:
[0032] The first step: in 5-isoquinolinesulfonic acid, first add catalyst N, N-dimethylformamide (DMF), then add thionyl chloride, wherein, the quality of 5-isoquinolinesulfonic acid and catalyst The ratio is 10:1, stirring, heating at 80°C for reflux reaction for 1 hour, cooling to 60°C, recovering thionyl chloride by distillation under reduced pressure, and further removing residual thionyl chloride by dichloromethane beating to obtain 5- Isoquinolinesulfonyl chloride hydrochloride (A);
[0033] Step 2: In dichloromethane, first add 5-isoquinolinesulfonyl chloride hydrochloride (A), then add water, slowly add sodium bicarbonate while stirring, and adjust the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com