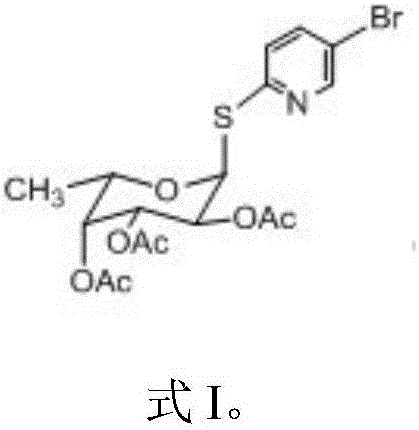
2, 3, 4-triacetyl-1-(5-bromo-2-pyridyl) sulfydryl-ahpha-L-fucose pyranoside
A fucopyranoside and triacetyl technology, which is applied in the preparation of sugar derivatives, sugar derivatives, and sugar derivatives, can solve the problems of unstable intermediates, cumbersome synthetic routes, and small steric hindrance, and achieves The effect of changing stereoselectivity, simple synthesis method, and cheap starting materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Preparation of 2,3,4-triacetyl-1-(5-bromo-2-pyridyl)mercapto-α-L-fucopyranoside
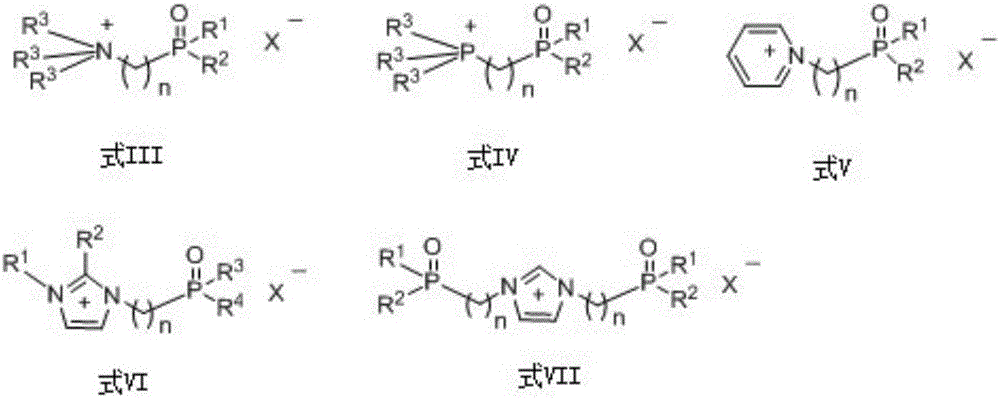
[0039] Weigh 0.486 g of FeCl 3 , 0.33 g of tetraacetylated fucose, and 0.19 g of 5-bromo-2-mercaptopyridine were placed in parallel reaction tubes. Add 10mL dichloromethane and ionic liquid PFIL-1[R 1 , R 2 , R 3 =ethyl, n=3, X=(CF 3 SO 2 ) 2 N] 0.5mL, airtight reaction test tube, stirring reaction, microwave irradiation using intermittent, power 350W, each irradiation 5 minutes, stop irradiation for 1 minute. Reaction was carried out for 5 hours, and the temperature was controlled at about 40 degrees Celsius. After the reaction was completed, water was added to terminate the reaction. After separation by silica gel chromatography, 0.25 g of Glycoside 1 was obtained with a yield of 50%. Mass spectrum (positive ion mode): m / z 462.02 (M+H) + . The ionic liquid can be reused without significant change in yield.
Embodiment 2
[0040] Example 2: Preparation of 2,3,4-triacetyl-1-(5-bromo-2-pyridyl)mercapto-α-L-fucopyranoside
[0041] 0.33 g of tetraacetylated fucose and 0.19 g of 5-bromo-2-mercaptopyridine were placed in parallel reaction tubes. Add 10mL trichloromethane and ionic liquid PFIL-3[R 1 , R 2 =phenyl, n=3, X=(CF 3 SO 2 ) 2 N] 1mL and 0.345mL of anhydrous SnCl 4 , airtight reaction test tube, stirring reaction, microwave irradiation using intermittent, power 400W, each irradiation 5 minutes, stop irradiation for 1 minute. Reaction was carried out for 3 hours, and the temperature was controlled at about 60 degrees Celsius. After the reaction was completed, water was added to terminate the reaction. After separation by silica gel column, 0.258 g of Glycoside 1 was obtained with a yield of 56%. The ionic liquid can be reused without significant change in yield.
Embodiment 3
[0042] Example 3: Preparation of 2,3,4-triacetyl-1-(5-bromo-2-pyridyl)mercapto-α-L-fucopyranoside
[0043] Weigh 0.591 g of Nd(OTf) respectively 3 , 0.33 g of tetraacetylated fucose, and 0.19 g of 5-bromo-2-mercaptopyridine were placed in parallel reaction tubes. Add 10mL of 1,2-dichloroethane and ionic liquid PFIL-2[R 1 , R 2 , R 3 =phenyl, n=4, X=(CF 3 SO 2 ) 2 N] 0.5mL, airtight reaction test tube, stirring reaction, microwave irradiation using intermittent, power 500W, each irradiation 5 minutes, stop irradiation for 1 minute. Reaction was carried out for 5 hours, and the temperature was controlled at about 80 degrees Celsius. After the reaction was completed, water was added to terminate the reaction. After separation by silica gel column, 0.336 g of Glycoside 1 was obtained with a yield of 73%. The ionic liquid can be reused without significant change in yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com