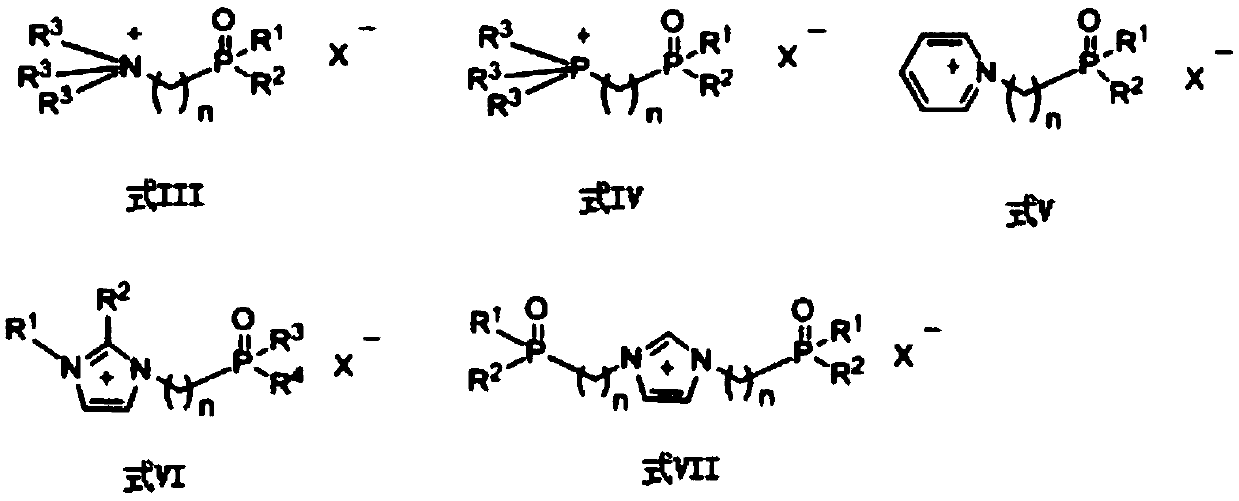
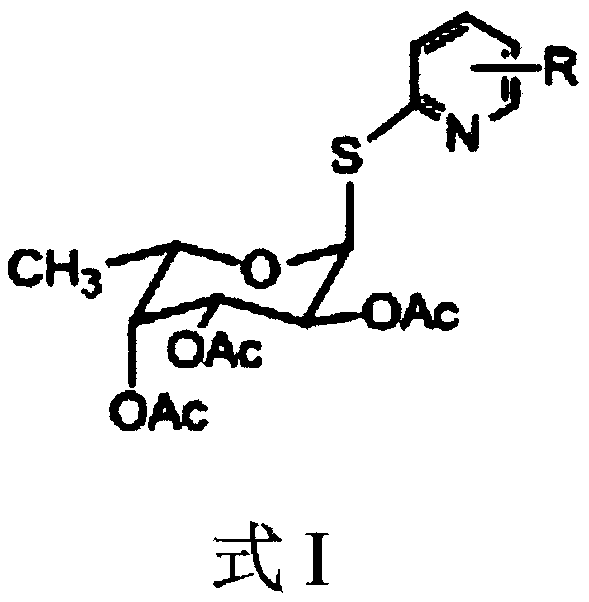
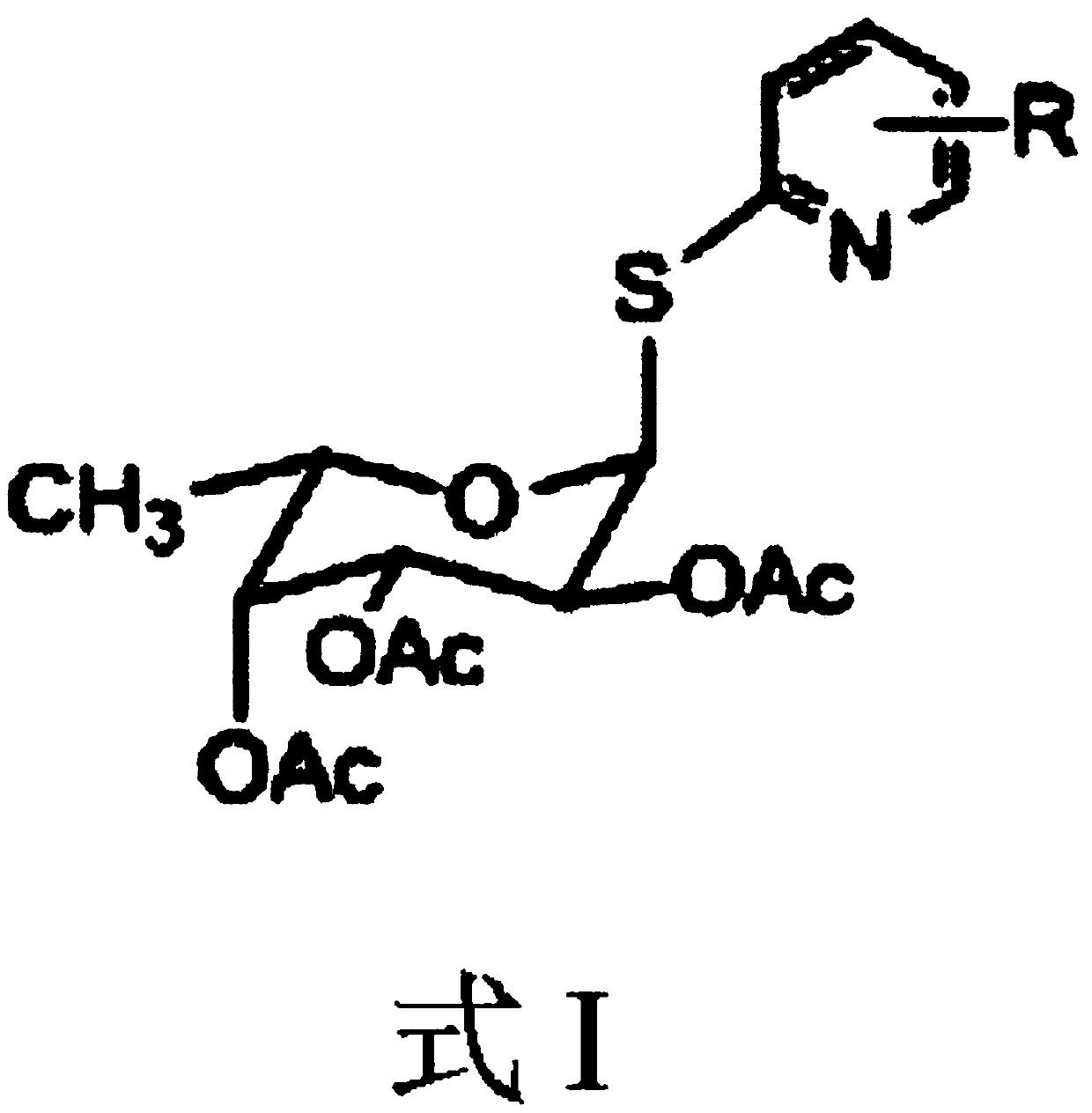
2,3,4-Triacetyl-1-(nitro-2-pyridyl)mercapto-α-l-fucopyranoside
A technology of fucopyranoside and triacetyl, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of unstable intermediates, cumbersome synthetic routes, and small steric hindrance, and achieve Effects of changing stereoselectivity, simple synthesis methods, and cheap starting materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Preparation of 2,3,4-triacetyl-1-(3-nitro-2-pyridyl)mercapto-alpha-L-fucopyranoside
[0041] Weigh 0.486 g of FeCl3, 0.33 g of tetraacetylated fucose, and 0.155 g of 3-nitro-2-mercaptopyridine into parallel reaction tubes. Add 10 mL of dichloromethane and 0.5 mL of ionic liquid PFIL-1 [R1, R2, R3 = ethyl, n = 3, X = (CF3SO2)2N] under a nitrogen atmosphere, seal the reaction test tube, stir the reaction, and microwave irradiation using Intermittent, power 350W, irradiating for 5 minutes each time, stop irradiating for 1 minute. Reaction was carried out for 5 hours, and the temperature was controlled at about 40 degrees Celsius. After the reaction was completed, water was added to terminate the reaction. After separation by silica gel column, 0.21 g of Glycoside 1-1 was obtained with a yield of 49%. The ionic liquid can be reused without significant change in yield.
Embodiment 2
[0042] Example 2: Preparation of 2,3,4-triacetyl-1-(3-nitro-2-pyridyl)mercapto-alpha-L-fucopyranoside
[0043] 0.33 g of tetraacetylated fucose and 0.155 g of 3-nitro-2-mercaptopyridine were placed in parallel reaction tubes. Under nitrogen atmosphere, add 10 mL of chloroform, 1 mL of ionic liquid PFIL-3 [R1, R2 = phenyl, n = 3, X = (CF3SO2)2N] and 0.345 mL of anhydrous SnCl4, seal the reaction tube, stir the reaction, microwave Irradiation is intermittent, with a power of 400W, irradiating for 5 minutes each time, and stopping the irradiation for 1 minute. Reaction was carried out for 3 hours, and the temperature was controlled at about 60 degrees Celsius. After the reaction was completed, water was added to terminate the reaction. After separation by silica gel column, 0.24 g of Glycoside 1-1 was obtained with a yield of 55%. The ionic liquid can be reused without significant change in yield.
Embodiment 3
[0044] Example 3: Preparation of 2,3,4-triacetyl-1-(3-nitro-2-pyridyl)mercapto-alpha-L-fucopyranoside
[0045] Weigh 0.591 g of Nd(OTf)3, 0.33 g of tetraacetylated fucose, and 0.155 g of 3-nitro-2-mercaptopyridine into parallel reaction tubes. Add 10 mL of 1,2-dichloroethane and 0.5 mL of ionic liquid PFIL-2 [R1, R2, R3 = phenyl, n = 4, X = (CF3SO2)2N] under a nitrogen atmosphere, seal the reaction tube, and stir the reaction. The microwave irradiation adopts intermittent type, the power is 500W, each irradiation is 5 minutes, and the irradiation is stopped for 1 minute. Reaction was carried out for 5 hours, and the temperature was controlled at about 80 degrees Celsius. After the reaction was completed, water was added to terminate the reaction. After separation by silica gel column, 0.25 g of Glycoside 1-1 was obtained with a yield of 59%. The ionic liquid can be reused without significant change in yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com