Methylpyrane anthocyanin preparation method and application of methylpyrane anthocyanin
A technology of methylpyranocyanide and formic acid, which is applied in the field of preparation of methylpyranocyanin, can solve the problems of low synthesis yield and slow synthesis speed, and achieve the effects of increasing yield, promoting synthesis, and shortening reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] After mixing absolute ethanol and distilled water at a volume ratio of 1:9, a chromatographic grade formic acid solution with a volume fraction of 0.2% is added dropwise to obtain the first product, the pH of which is less than 7.
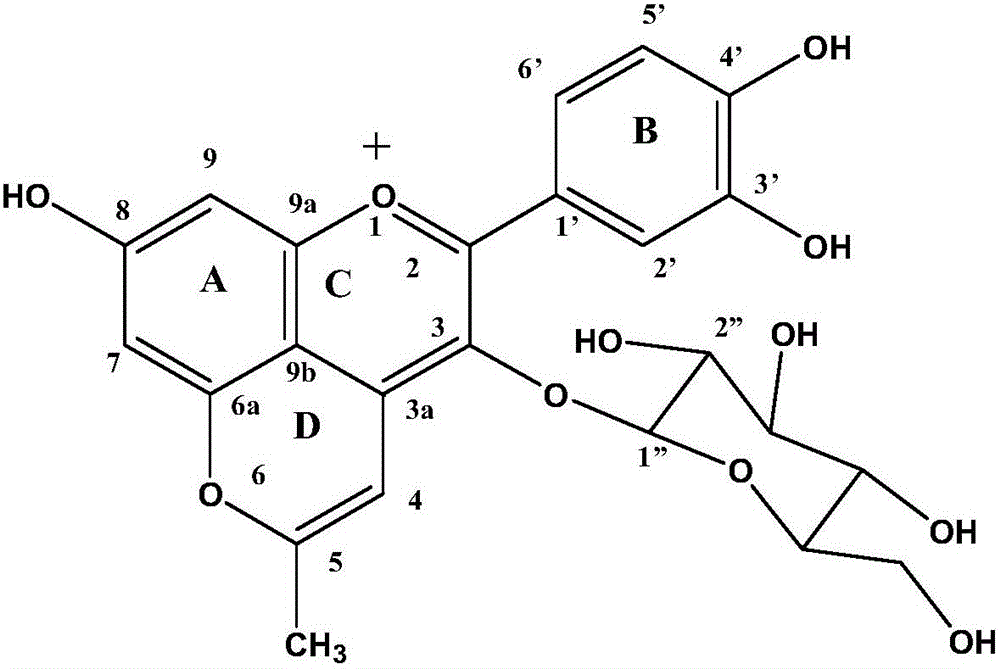
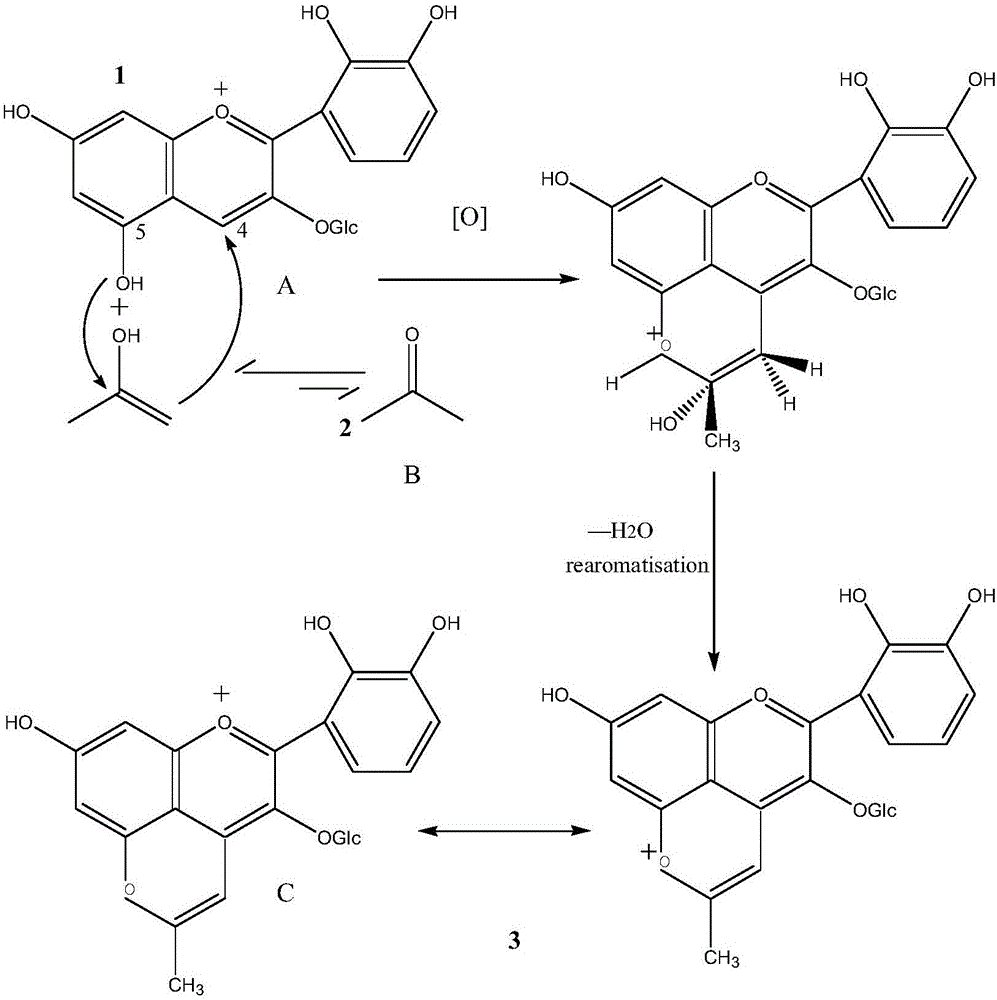
[0034] Weigh 1mg cyanidin 3-oxyglucoside and dissolve it in the first product 10ml, continue to add 1ml of chromatographic grade acetone to the reaction system to obtain the second product 1, in the second product 1, cyanidin 3-oxyglucoside: Acetone = 1:1.
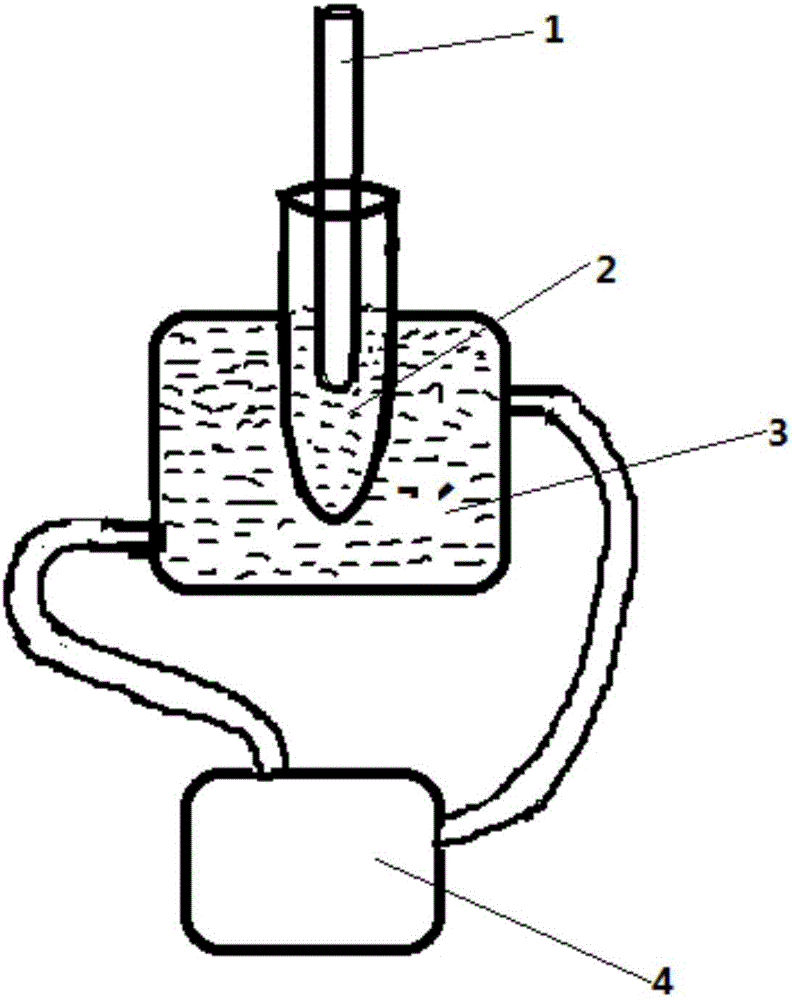
[0035] The second product 1 was placed in the dark condition figure 2 The third product 1 is obtained by ultrasonic treatment in the described device. Wherein, during ultrasonic treatment, the horn is placed at 1cm below the liquid surface; the ultrasonic power of ultrasonic treatment is 100W, the total time of ultrasonic treatment is 20min, and the temperature of ultrasonic treatment is 35°C; the duty ratio of ultrasonic treatment is 1: 1. The time for a single ultrasonic treatment i...
Embodiment 2
[0038] After mixing absolute ethanol and distilled water at a volume ratio of 1:9, a chromatographic grade formic acid solution with a volume fraction of 0.2% is added dropwise to obtain the first product, the pH of which is less than 7.
[0039] Weigh 1mg cyanidin 3-oxyglucoside and dissolve it in the first product 10ml, continue to add 1ml chromatographic grade acetone to the reaction system to obtain the second product 2, in the second product 2, cyanidin 3-oxyglucoside: Acetone = 1:1.
[0040] The second product 2 was placed in the figure 2 Ultrasonic treatment in the described device yields the third product 2. Wherein, during ultrasonic treatment, the horn is placed at 1cm below the liquid surface; the ultrasonic power of ultrasonic treatment is 100W, the total time of ultrasonic treatment is 40min, and the temperature of ultrasonic treatment is 35°C; the duty ratio of ultrasonic treatment is 1: 1. The time for a single ultrasonic treatment is 1s, that is, after ultra...
Embodiment 3
[0043] After mixing absolute ethanol and distilled water at a volume ratio of 1:9, a chromatographic grade formic acid solution with a volume fraction of 0.2% is added dropwise to obtain the first product, the pH of which is less than 7.
[0044] Weigh 1mg cyanidin 3-oxyglucoside and dissolve it in the first product 10ml, continue to add 1ml chromatographic grade acetone to the reaction system to obtain the second product 3, in the second product 3, cyanidin 3-oxyglucoside: Acetone = 1:1.
[0045] The second product 3 was placed in the figure 2 Ultrasonic treatment in the described device yields the third product 3. Wherein, during ultrasonic treatment, the horn is placed at 1cm below the liquid surface; the ultrasonic power of ultrasonic treatment is 100W, the total time of ultrasonic treatment is 60min, and the temperature of ultrasonic treatment is 35°C; the duty ratio of ultrasonic treatment is 1: 1. The time for a single ultrasonic treatment is 1s, that is, after ultra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com