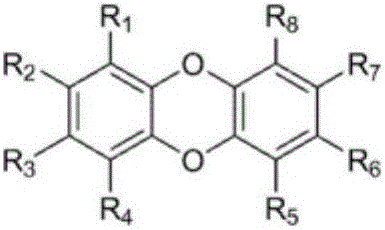
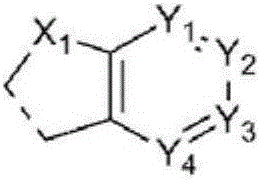
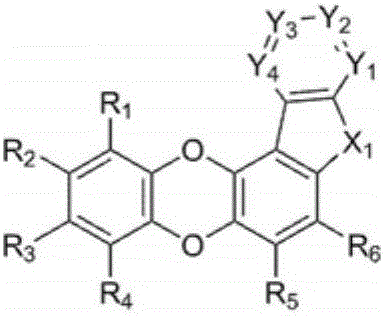
Organic compound and organic electroluminescent element comprising same
一种化合物、化学式的技术,应用在新型有机化合物及包含其的有机电致发光元件领域,能够解决热稳定性降低、玻璃化转变温度低等问题,达到效率提高、发光能力优异的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0202] [Synthesis Example 1] Synthesis of Cpd 1
[0203]
[0204] The compound Inv1 (2.7g, 10.0mmol), N-(4'-bromo-[1,1'-biphenyl]-4-yl)-N-phenylnaphthalene-2-amine synthesized in Preparation Example 1 (5.4g, 12.0mmol) was dissolved in 100ml of toluene, then Pd was added under nitrogen 2 (dba) 3 (0.9g, 1.0mmol). After adding NaOtBu (2.9g, 30mmol) to it, (t-Bu) 3 P (1.0 ml, 1.0 mmol) was put into the above reaction solution, and then the mixture was refluxed and stirred for 5 hours.
[0205] After confirming the completion of the reaction by TLC, it was cooled to normal temperature. After the reaction, distilled water was added, and the organic layer was extracted with ethyl acetate. Use Na 2 SO 4 After drying and distillation under reduced pressure, it was purified by column chromatography to obtain compound Cpd 1 (5.5 g, yield 86%).
[0206] HRMS[M] + :642.230
Synthetic example 2
[0207] [Synthesis Example 2] Synthesis of Cpd 2
[0208] Use 7-bromo-9,9-dimethyl-N-(naphthalene-1-yl)-N-phenyl-9H-fluoren-2-amine (5.9g, 12.0mmol) instead of the one used in Synthesis Example 1. N-(4'-bromo-[1,1'-biphenyl]-4-yl)-N-phenylnaphthalene-2-amine, except that it was obtained by implementing the same process as in Synthesis Example 1 above The target compound Cpd 2 (5.8 g, yield 85%).
[0209] HRMS[M] + :682.262
Synthetic example 3
[0210] [Synthesis Example 3] Synthesis of Cpd 3
[0211] Use N,N-di([1,1'-biphenyl]-4-yl)-4'-bromo-[1,1'-biphenyl]-4-amine (6.6g, 12.0mmol) instead In addition to the N-(4'-bromo-[1,1'-biphenyl]-4-yl)-N-phenylnaphthalene-2-amine used in Synthesis Example 1, it was synthesized as described above The target compound Cpd 3 (6.7 g, yield 85%) was obtained by the same process in Example 1.
[0212] HRMS[M] + :744.277
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com