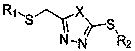
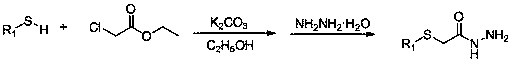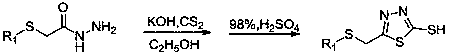Disulfide derivatives containing 1,3,4-oxa(thia)diazole, its preparation method and application
A technology of bissulfides and thiadiazoles, which is applied in the field of preparation of disulfide derivatives, can solve problems such as instability of sulfone compounds, difficulty in practical application, and increased cost, and achieve simple structure, low production cost, The effect of simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The synthesis of embodiment 1, 2-((4-fluorophenylthio)methyl)-5-ethylthio-1,3,4-oxadiazole (compound number is A1), comprises the following steps:
[0057] Step 1: Put substituted 4-fluorothiophenol and potassium carbonate into a 100mL three-necked round-bottomed flask, use absolute ethanol as solvent, stir for 5 minutes, then add ethyl chloroacetate dropwise, then react at room temperature for 6-8 h, TLC tracking the reaction process, after the raw material point disappeared, 80% hydrazine hydrate was added dropwise, and then refluxed for 6-8 h (molar ratio: 4-fluorothiophenol: potassium carbonate: ethyl chloroacetate: hydrazine hydrate=1 :1.4:1:2), reflux reaction, TLC to follow the reaction process, after the disappearance of the raw material point, stop the reaction, distill the ethanol off under reduced pressure, cool and stand still, wash with water and filter with suction to obtain white crystals, which are recrystallized with ethanol to obtain 4 - Fluorophenylth...
Embodiment 2
[0060] Example 2, the synthesis of 2-((4-fluorophenylthio)methyl)-5-methylthio-1,3,4-oxadiazole (compound number is A2), comprises the following steps:
[0061] Put the intermediate 2-mercapto-5-(4-fluorophenylthio)methyl-1,3,4-oxadiazole and sodium hydroxide obtained in the second step of Example 1 into a 100mL three-necked round-bottomed flask , and water as a solvent, after the solid is completely dissolved, add dropwise dimethyl sulfate (molar ratio: 2-mercapto-5-(4-fluorophenylthio)methyl-1,3,4-oxadiazole : sodium hydroxide: dimethyl sulfate = 1:1.3:1), then stirred at room temperature, followed the reaction process by TLC, after the disappearance of the raw material point, stopped the reaction, extracted with dichloromethane (30 mL×3), combined the organic layers, Dry with anhydrous magnesium sulfate, filter, and evaporate the solvent under reduced pressure to obtain a yellow transparent oily liquid, which is purified by column chromatography (V 石油醚 :V 乙酸乙酯 =3:1) to ob...
Embodiment 3
[0062] Example 3, the synthesis of 2-((4-fluorophenylthio)methyl)-5-benzylthio-1,3,4-oxadiazole (compound number is A3), comprises the following steps:
[0063] Put the intermediate 2-mercapto-5-(4-fluorophenylthio)methyl-1,3,4-oxadiazole and sodium hydroxide obtained in the second step of Example 1 into a 100mL three-necked round-bottomed flask , and water as a solvent, after the solid is completely dissolved, benzyl chloride (molar ratio: 2-mercapto-5-(4-fluorophenylthio)methyl-1,3,4-oxadiazole:hydrogen Sodium oxide: benzyl chloride = 1:1.3:1), then stirred at room temperature, followed the reaction process by TLC, stopped the reaction after the disappearance of the raw material point, extracted with dichloromethane (30 mL×3), combined the organic layers, and washed with anhydrous sulfuric acid Dried over magnesium, filtered, evaporated the solvent under reduced pressure to obtain a solid, filtered and dried, and recrystallized with absolute ethanol to obtain 2-((4-fluorophe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com