Iridium(iii) complexes based on 2-(4-(dimidylboron)phenyl)pyridine ligands and their preparation methods and applications
A technology of micky boron and complexes, which is applied in the field of photocatalysts, can solve the problems that the excited state is difficult to be quenched by the catalyst, the molar extinction coefficient is low, and the stability is not good, so as to achieve excellent photochemical performance, high hydrogen output efficiency and long life. long effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
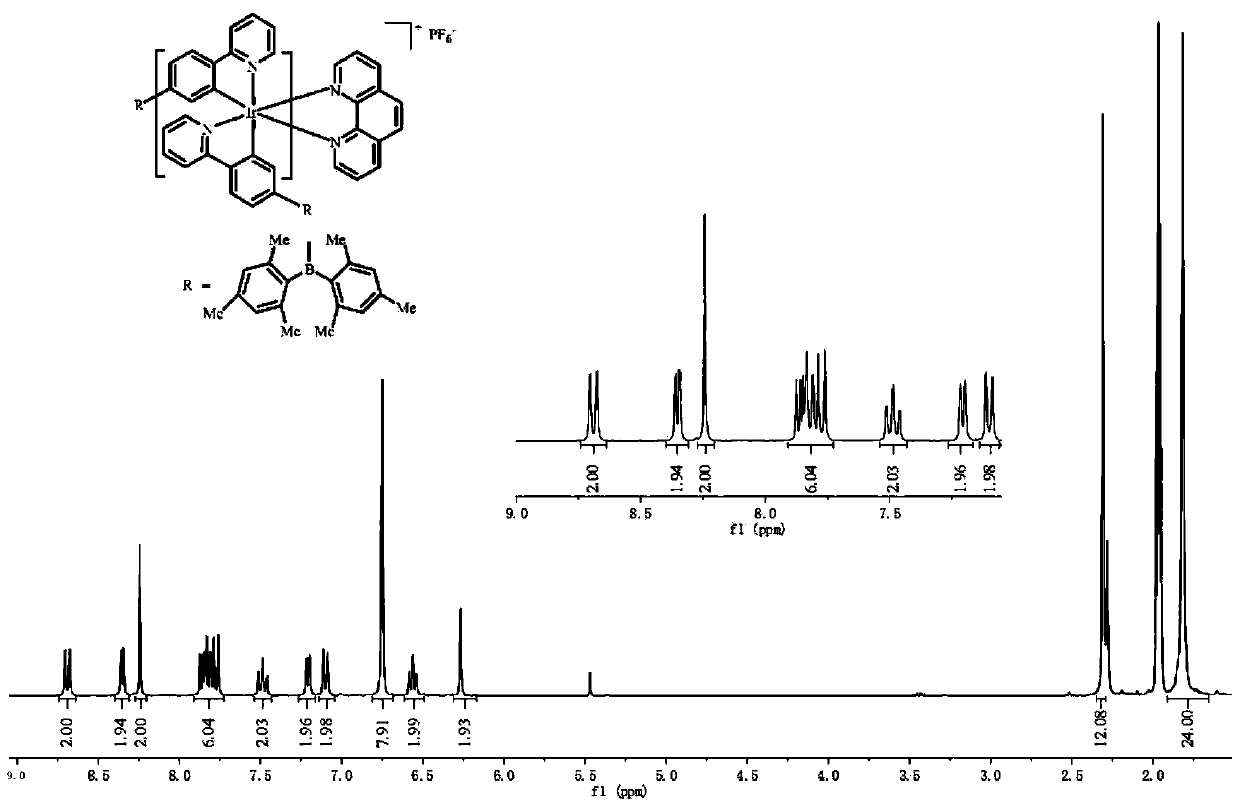
[0029] Preparation of iridium (III) complex intermediate: 853 mg of ligand 2-(4-(dimethylboron) phenyl) pyridine and 223 mg of iridium chloride hydrate were added to 60 mL of aqueous solution of 2-ethoxyethanol (2- Ethoxyethanol:water=3:1), in N 2 Reflux at 140°C for 24h in the atmosphere. The reaction was stopped, cooled, filtered, washed with 10 mL of water, 10 mL of ethanol, and 10 mL of acetone, and dried to obtain an orange-red powder.
[0030] Mix 418mg of iridium(III) complex intermediate with bipyridyl[3,2-a:2',3'-c]phenazine (2.5eq), anhydrous sodium carbonate (10eq) and add 40mL of methanol and dichloromethane Solution (CH 3 OH:CH 2 Cl 2 =1:1), in N 2 Reflux at 80°C for 24 hours in the atmosphere; then add ammonium hexafluorophosphate (10eq) into the above reaction solution, and continue to reflux for about 3 hours. Dichloromethane and ethanol are used as eluents for chromatographic separation, and the iridium (III) complex is obtained through a recrystallizati...
Embodiment 2
[0033] The preparation of the iridium (III) complex intermediate is the same as in Example 1.
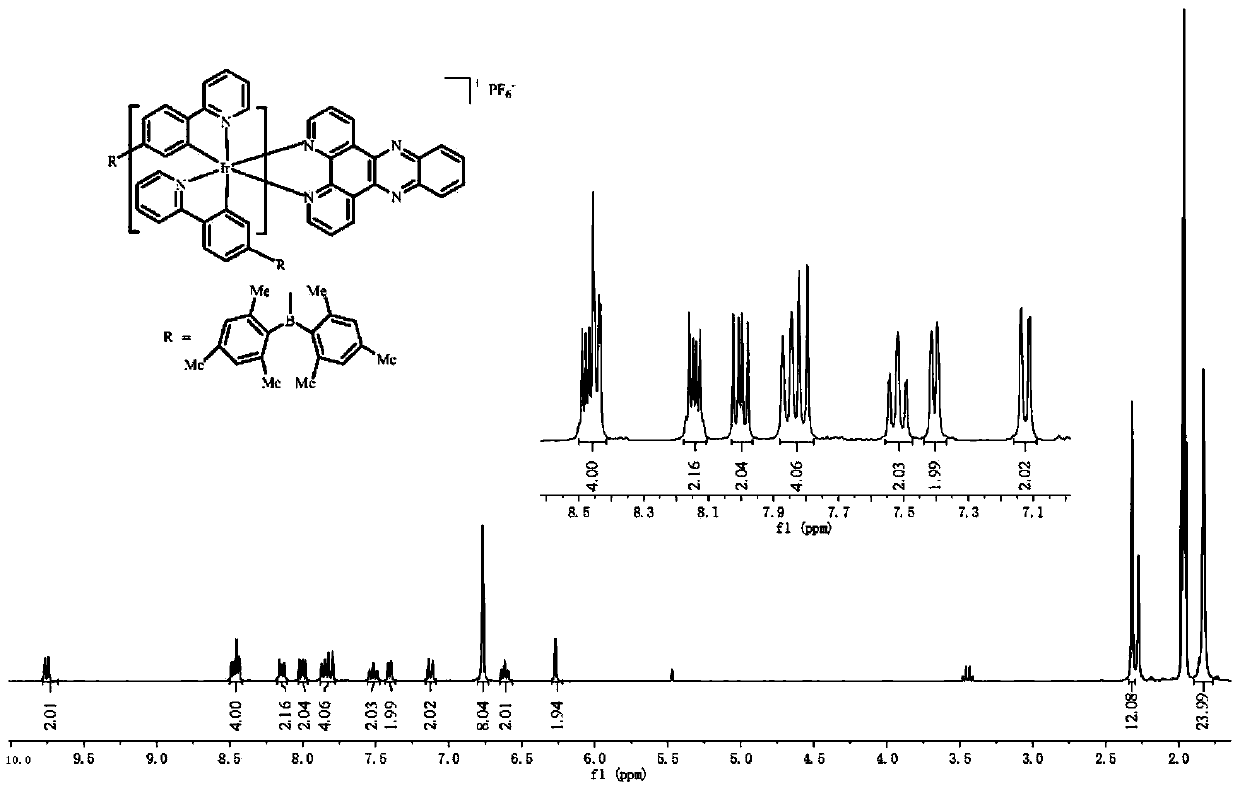
[0034] Add 300 mg of the intermediate of the Ir(III) complex with bipyridyl[3,2-f:2',3'-h]quinoxaline (2.5eq), anhydrous sodium carbonate (10eq) into 40mL of methanol and dichloro Methane mixed solution (CH 3 OH:CH 2 Cl 2 =1:1), in N 2 Reflux at 80°C for 24 hours in the atmosphere; then add ammonium hexafluorophosphate (10eq) into the above reaction solution, and continue to reflux for about 3 hours. Dichloromethane and ethanol are used as eluents for chromatographic separation, and the iridium (III) complex is obtained through a recrystallization process. Yield 250 mg (62%).
[0035] The iridium (III) complex is carried out nuclear magnetic resonance (H spectrogram sees figure 2 ), measured: 1 H NMR (300MHz, CD 3 CN)δ9.65(dd, J=8.3,1.3Hz,2H),9.23(s,2H),8.45(dt,J=12.9,6.5Hz,2H),8.00(dd,J=8.3,5.1Hz, 2H), 7.82(dd, J=13.8, 7.8Hz, 4H), 7.55–7.44(m, 2H), 7.32(d, J=5.4Hz, 2H), 7.1...
Embodiment 3
[0037] The preparation of the iridium (III) complex intermediate is the same as in Example 1.
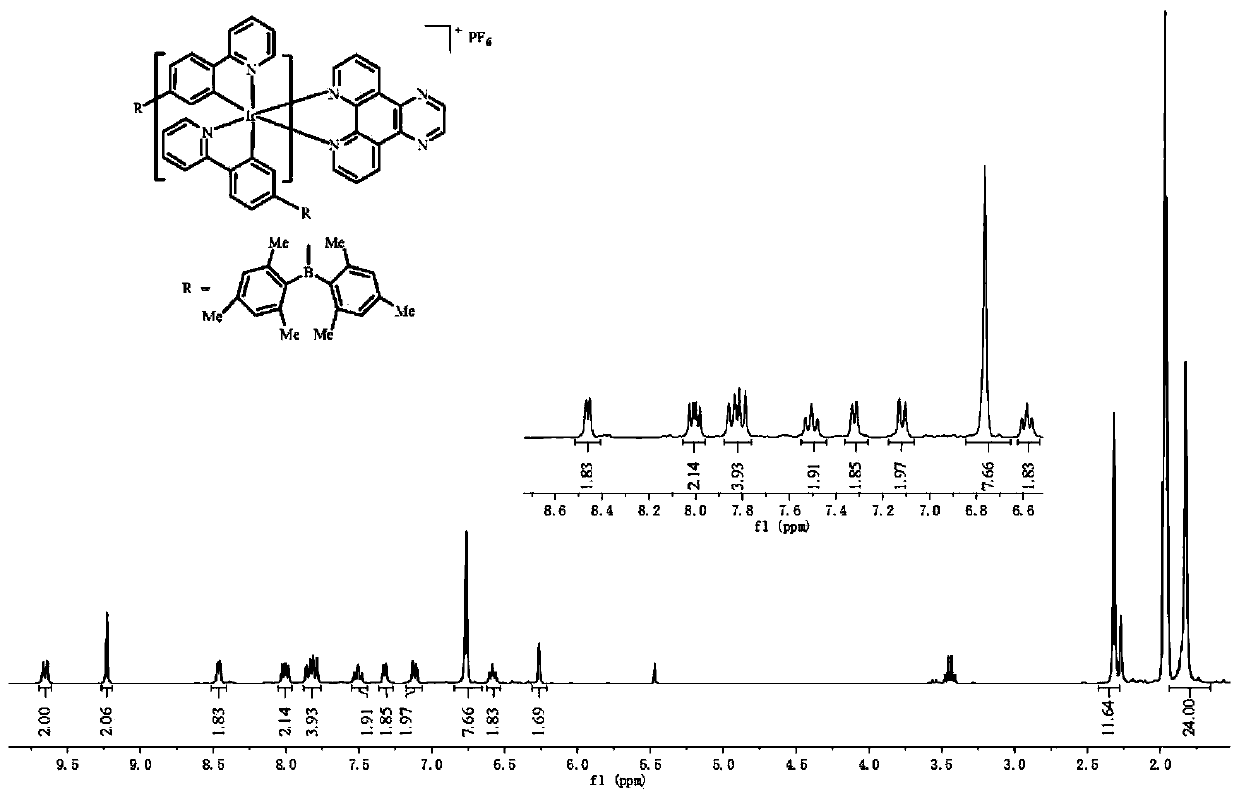
[0038] Add 410 mg of the intermediate of the iridium (III) complex, 1,10-phenanthroline (2.5 eq) and anhydrous sodium carbonate (10 eq) into a mixed solution of 40 mL of methanol and dichloromethane (CH 3 OH:CH 2 Cl 2 =1:1), in N 2 Reflux at 80°C for 24 hours in the atmosphere; then add ammonium hexafluorophosphate (10eq) into the above reaction solution, and continue to reflux for about 3 hours. Dichloromethane and ethanol are used as eluents for chromatographic separation, and the iridium (III) complex is obtained through a recrystallization process. Yield 380 mg (71%).
[0039] The iridium (III) complex is carried out nuclear magnetic resonance (H spectrogram sees image 3 ), measured: 1 H NMR (300MHz, CD 3 CN)δ8.69(dd,J=8.3,1.3Hz,2H),8.35(dd,J=5.0,1.3Hz,2H),8.24(s,2H),7.91–7.73(m,6H),7.54– 7.43(m,2H),7.21(d,J=5.2Hz,2H),7.10(dd,J=7.8,1.0Hz,2H),6.75(s,8H),6.61–6.49(m,2H),6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com