Drugs for diagnosis and treatment of Alzheimer's disease
An Alzheimer's disease and drug technology, applied in the field of neuroscience, can solve the problems of difficult brain biopsy, limited sensitivity and specificity, and lack of early diagnosis methods for AD.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 The experimental material and method that the present invention adopts
[0021] human subjects
[0022] This study complied with the Declaration of Helsinki and was approved by the Ethics Committee of Nanjing Medical University. The research objects come from healthy young people and old people among the active and retired employees of Nanjing First Hospital. All participants are Han Chinese and have no blood relationship with each other. Each participant signed a written consent form.
[0023] Purification and culture of monocytes from human circulating blood
[0024] According to the method described by Zondler and colleagues [6] , mononuclear cells were isolated from human venous whole blood. The general process is: use (Sigma-Aldrich Company) instrument, adopt density gradient centrifugation and CD-14 positive magnetic bead separation technology (Miltenyi Biotec Company) to realize cell separation. Resuspension of cells was performed in RPMI 1640...
Embodiment 2
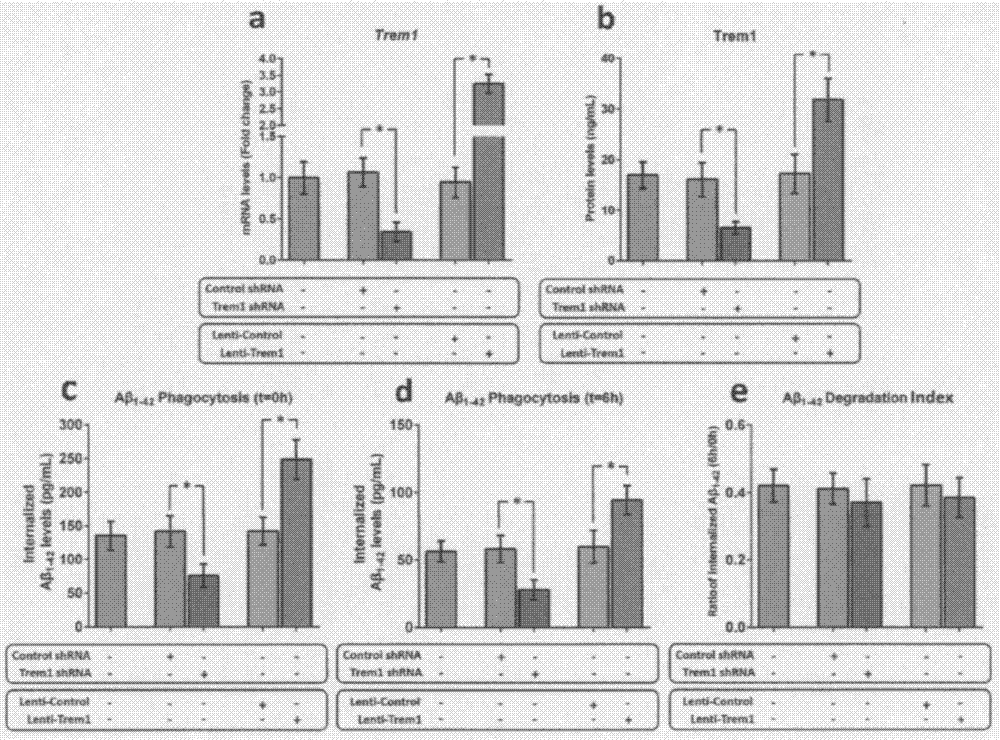
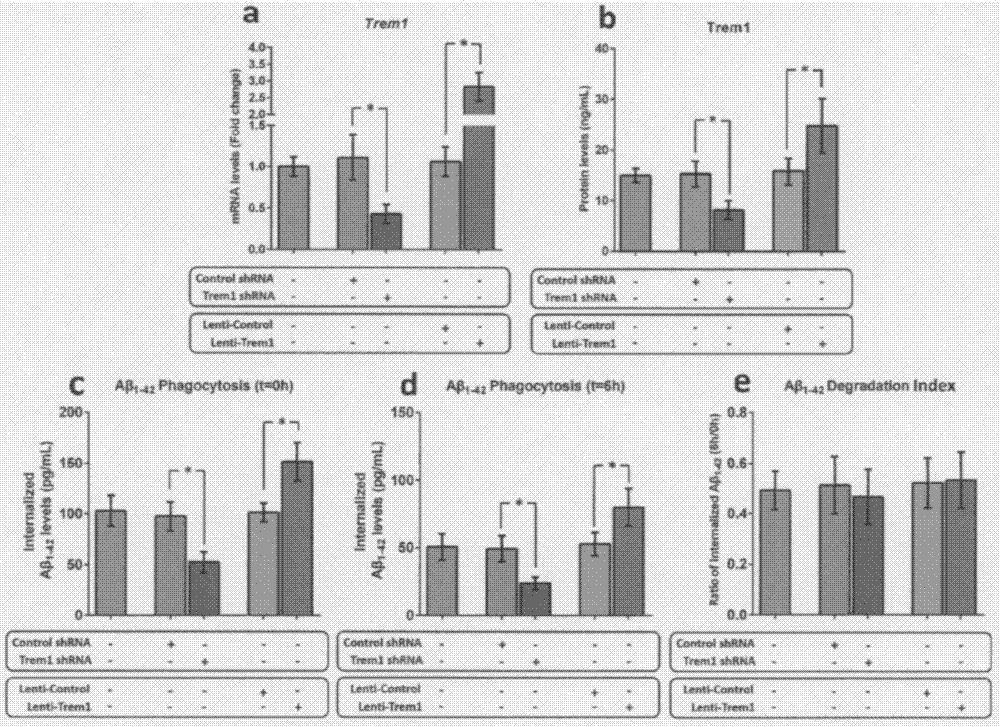
[0053] Example 2 Trem1 can regulate the phagocytosis of mouse microglia on Aβ
[0054] As the only mononuclear phagocytes in the central nervous system (CNS), microglia participate in the clearance of Aβ through phagocytosis. To clarify the role of TREM1 in the phagocytosis of Aβ by microglia, we manipulated the expression of Trem1 in microglia isolated from WT pups using a lentiviral mechanism. After 72 hours of transduction, the expression level of Trem1 mRNA and the change of Trem1 protein level were measured by qRT-PCR and ELISA technology, respectively. Afterwards, the in vitro test method provided by Griciuc et al. was used to detect the effect of microglia on Aβ 1-42 phagocytosis and degradation. Knockout of Trem1 leads to internalization of Aβ in microglia of young WT mice 1-42 levels decreased by 52% (P1-42 The level increased nearly 1.7 times (P1-42 degradation index.
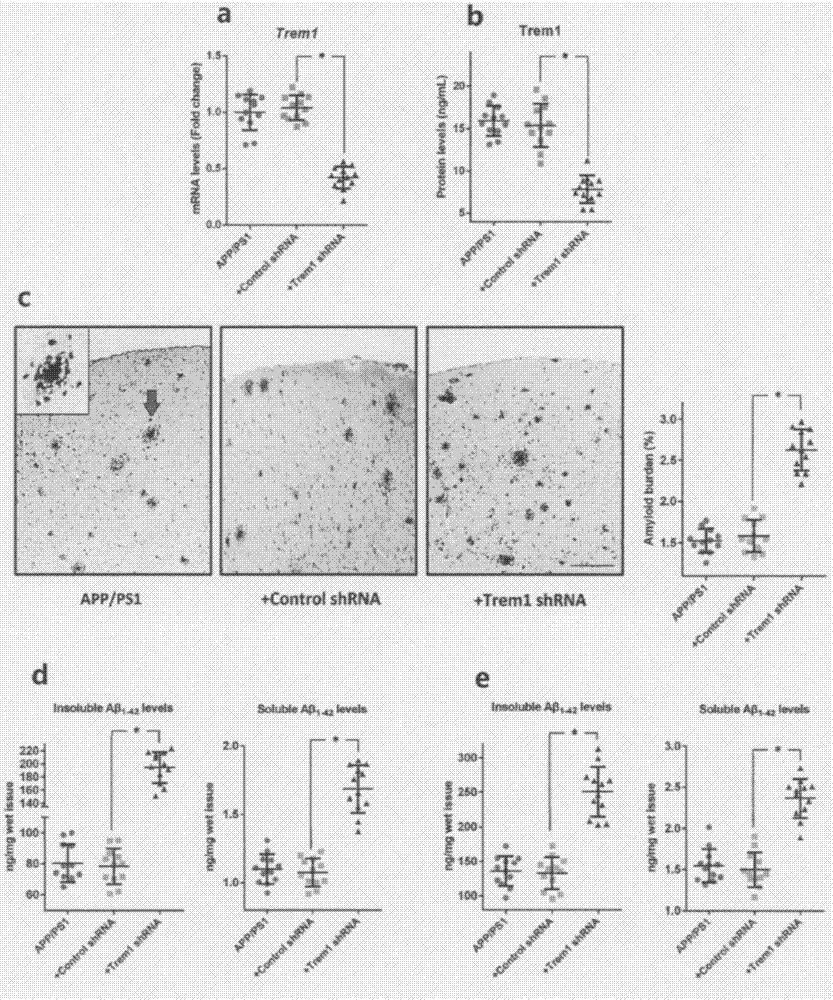
[0055] According to previous studies, APP / PSEN1 mice showed significant AD-related phenotypes ...
Embodiment 3
[0056] Example 3 During the progressive disease stage of APP / PSEN1 mice, the expression level of Trem1 on microglia is relatively stable.
[0057] Since TREM1 can promote microglia-mediated Aβ phagocytosis under AD conditions, we reasonably hypothesize that TREM1 may play a key role in AD progression. To test this hypothesis, we first explored the dynamic changes in the levels of Trem1 and its adapter protein Tyrobp in microglia in APP / PSEN1 mice at 1, 4, 7 and 10 months of age. During disease progression in APP / PSEN1 mice, the expression levels of Trem1 or Tyrobp did not change significantly (Trem1 mRNA level: P=0.31; Trem1 protein level: P=0.4; Tyrobp mRNA level: P=0.77). Meanwhile, we also did not find any significant differences in the expression levels of Trem1 or Tyrobp in microglia between APP / PSEN1 mice and their age-matched WT mice.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com