Competitive ELISA qualitative and quantitative detection method of oil adjuvant vaccine
A quantitative detection method and quantitative detection technology are applied in the field of foot-and-mouth disease synthetic peptide vaccine detection, which can solve the problems of no universal and accurate method for quantitative detection and can not respond well to effective antigen content, etc. The effect of detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
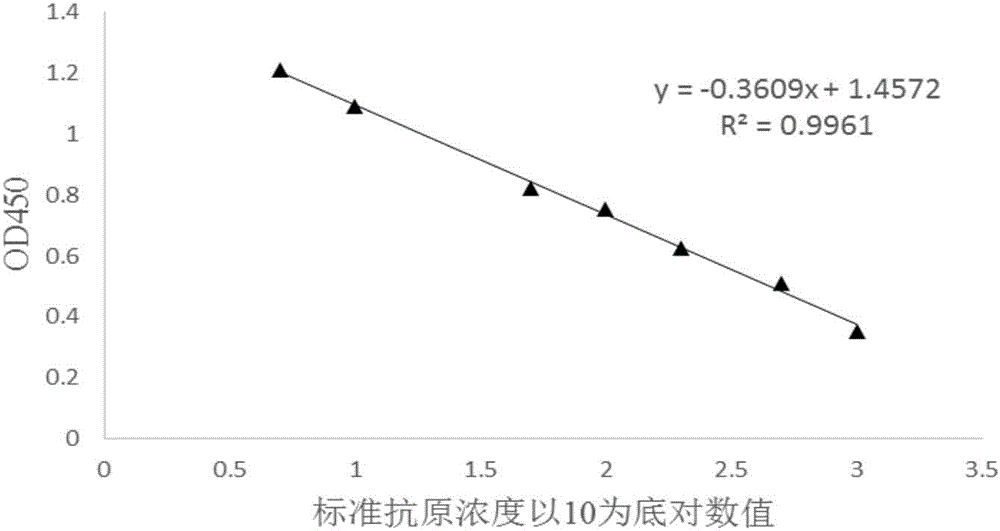
[0034] This embodiment provides a competitive ELISA qualitative and quantitative detection method for oil adjuvant vaccines, specifically using the following steps:
[0035] 1. Antigen coating: 3 μg / mL artificially synthesized FMD antigen, 100 μL per well, was immobilized onto the ELISA plate;
[0036] 2. Dilute the antibody: Dilute the antibody with known titer to a certain ratio, and the antibody titer after dilution is 1:250000;
[0037] 3. Dilution of the antigen to be tested (the antigen to be tested is a finished product of a synthetic peptide antigen, and the antigen concentration of the antigen to be tested is 39.25 μg / mL as measured by HPLC method) and the standard antigen: on the serum dilution plate, dilute the antigen to be tested with the diluent The test antigen was diluted 1:100, and the standard antigen was diluted according to the diluted concentrations of 1000ng / mL, 500ng / mL, 200ng / mL, 100ng / mL, 50ng / mL, 10ng / mL, and 5ng / mL;
[0038] 4. Antigen-antibody reac...
Embodiment 2
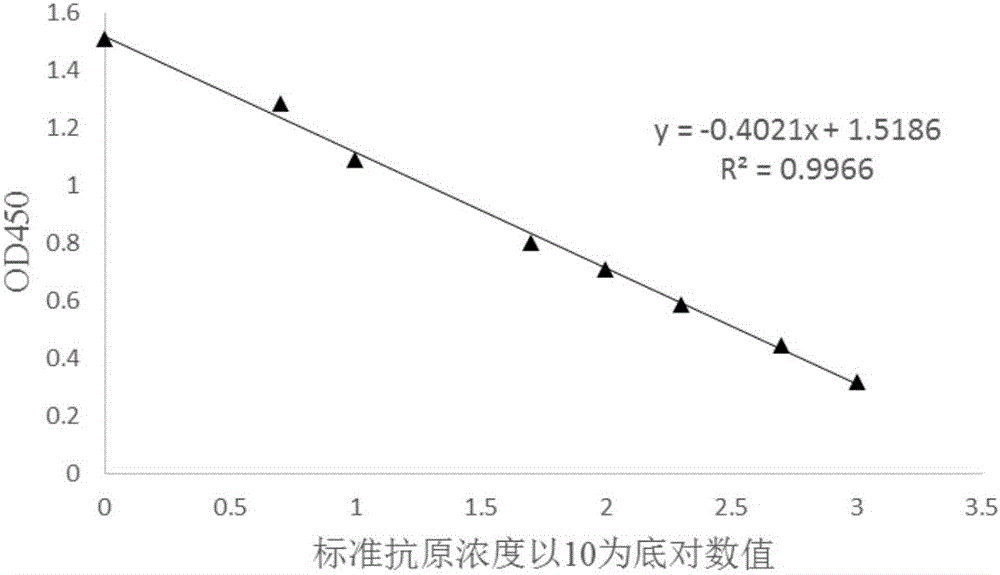
[0045] This embodiment provides a competitive ELISA qualitative and quantitative detection method for oil adjuvant vaccines, specifically using the following steps:
[0046] 1. Antigen coating: 3 μg / mL artificially synthesized FMD antigen, 100 μL per well, was immobilized onto the ELISA plate;
[0047] 2. Dilute the antibody: Dilute the antibody with known titer to a certain ratio, and the antibody titer after dilution is 1:1100000;
[0048] 3. Antigen to be tested (the antigen to be tested is the aqueous phase sample after vaccine demulsification, and the demulsification efficiency is 89.2%, after purification, adopting HPLC method to record its antigen concentration is 148.83ng / mL) and standard antigen dilution : On the serum dilution plate, dilute the antigen to be tested with the diluent at 1:100, and dilute the known standard antigen. The dilution concentrations are 1000ng / mL, 500ng / mL, 200ng / mL, 100ng / mL, 50ng / mL , 10ng / mL, 5ng / mL, 1ng / mL;
[0049] 4. Antigen-antibody ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Demulsification efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com