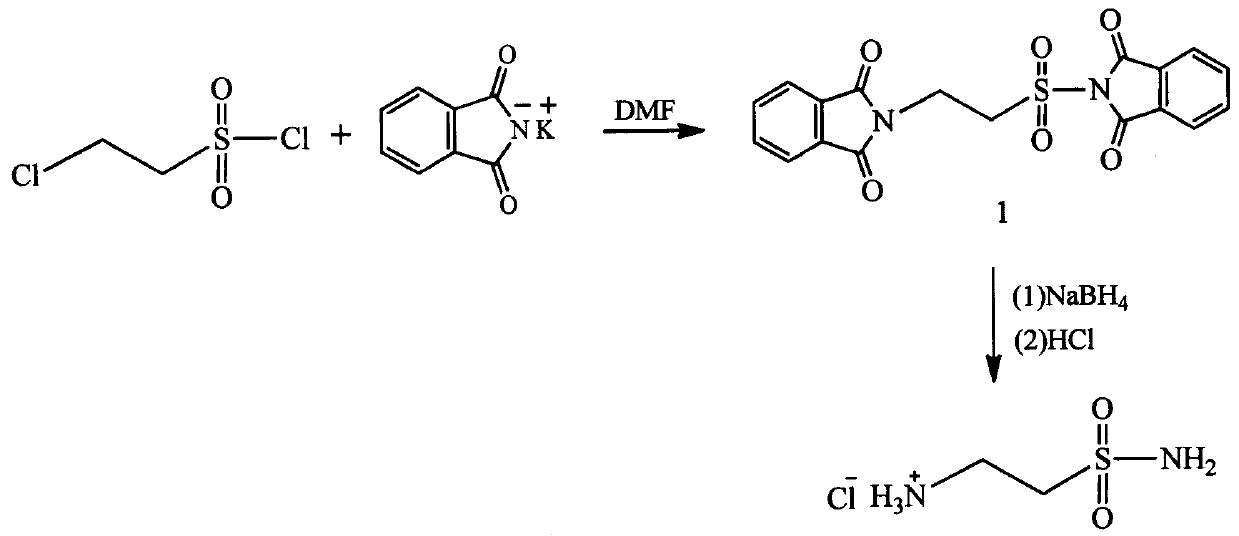
A kind of method preparing 2-aminoethylsulfonamide hydrochloride
A technology of aminoethylsulfonamide and hydrochloride, applied in the preparation of sulfonic acid amide, organic chemistry and other directions, can solve problems such as complex preparation process, and achieve the effects of simple preparation process, no discharge of three wastes, and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Preparation of intermediate 1
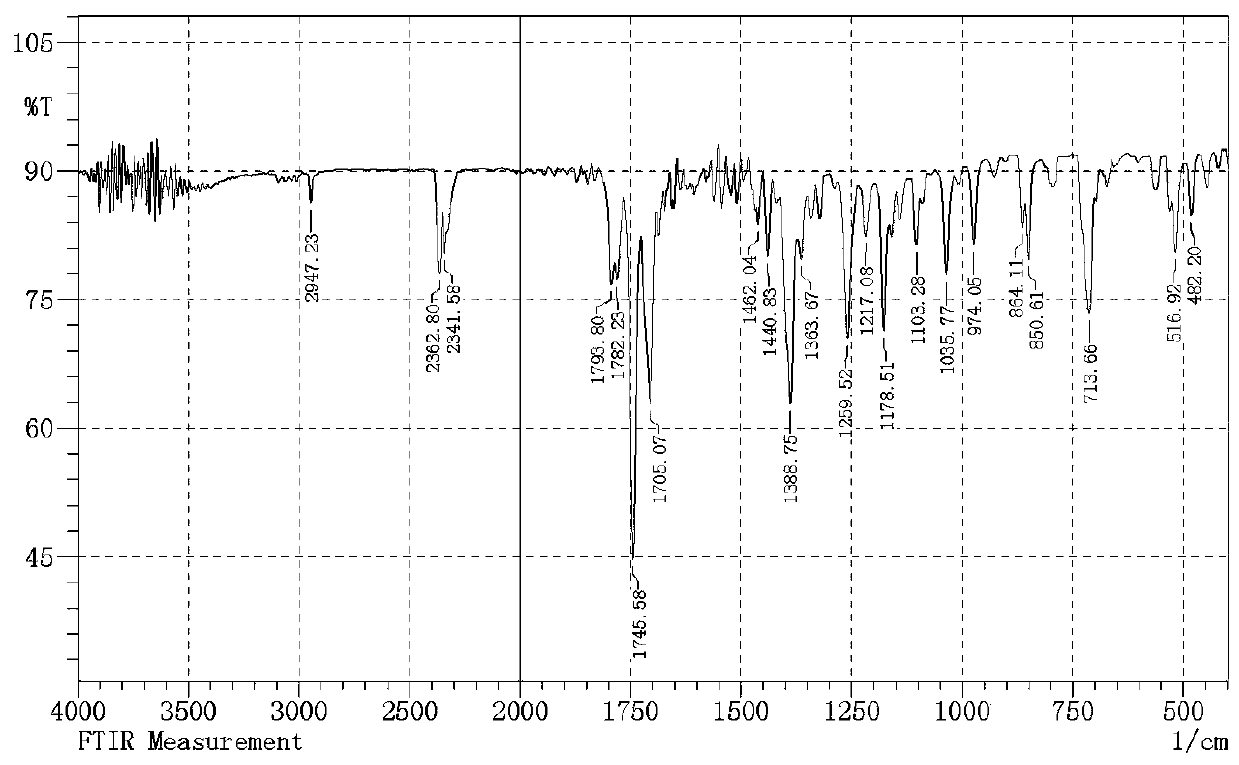
[0021] Add 55.56 g (0.3 mol) of potassium phthalimide and 300 ml of N,N-dimethylformamide into a 500 ml three-necked flask, and add 16.3 chloroethylsulfonyl chloride dropwise at room temperature gram (0.1 mol), the dropping speed kept the reaction temperature not exceeding 60°C, and the dropwise addition was completed within 1 hour; after reacting at room temperature for 30 hours, suction filtered, and vacuum-dried at 60°C for 10 hours to obtain 52.86 grams of white solid intermediate 1 , the yield was 91.77 %; melting point: 239.1-239.7 ℃; IR (KBr pellet, cm −1 ): 2947.2, 2362.8, 2341.5, 1793.8, 1782.2, 1745.5, 1705.1, 1462, 1440.8,1388.7, 1363.6, 1259.5, 1217.1, 1178.5, 1103.2,1035.7, 974, 864.1, 850.6,713.6, 563.2, 516.2, 482.2; 1 H NMR (400 MHz, DMSO-D 6 ) δ(ppm): 7.91-7.80(m, 4H),7.79-7.74(m, 4H), 4.11(s, 4H); HRMS (ESI) m / z 385.0517 (M+H) + .
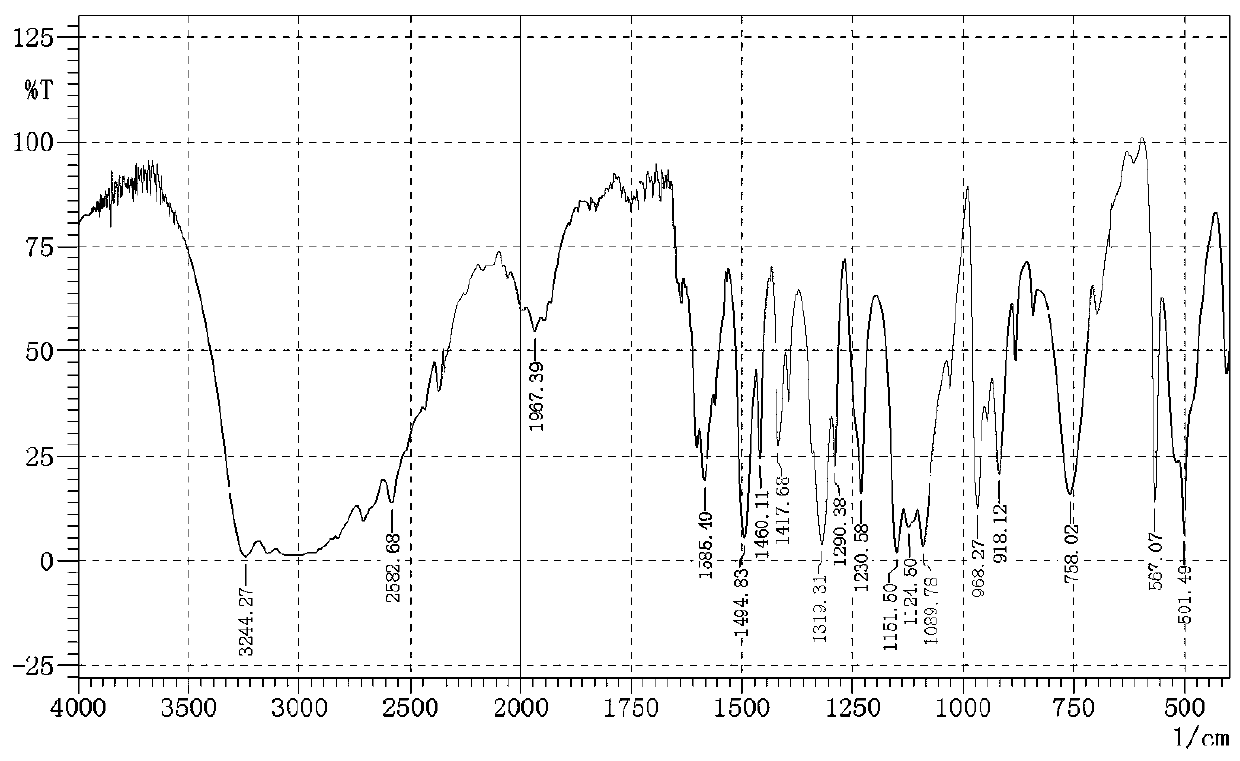
[0022] (2) Preparation of 2-aminoethylsulfonamide hydrochloride
[0023] 38.4 gr...
Embodiment 2
[0025] (1) Preparation of intermediate 1
[0026] Add 55.56 g (0.3 mol) of potassium phthalimide and 300 ml of N,N-dimethylformamide into a 500 ml three-necked flask, and add 24.45 chloroethylsulfonyl chloride dropwise at room temperature gram (0.15 mol), the rate of addition kept the reaction temperature not exceeding 60°C, and the dropwise addition was completed within 1 hour. After reacting at room temperature for 10 hours, suction filtration, and vacuum drying at 60°C for 10 hours gave 51.59 grams of white solid intermediate 1 , the yield was 89.57 %; melting point: 239.1-239.7 ℃; IR (KBr pellet, cm −1 ): 2947.2, 2362.8, 2341.5, 1793.8, 1782.2, 1745.5, 1705.1, 1462,1440.8, 1388.7, 1363.6, 1259.5, 1217.1, 1178.5, 1103.2,1035.7, 974, 864.1,850.6, 713.6, 563.2, 516.2, 482.2; 1 H NMR (400 MHz, DMSO-D 6 ) δ(ppm): 7.91-7.80(m, 4H), 7.79-7.74(m, 4H), 4.11(s, 4H); HRMS (ESI) m / z 385.0517 (M+H) + .
[0027] (2) Preparation of 2-aminoethylsulfonamide hydrochloride
[0028]38.4 ...
Embodiment 3
[0030] (1) Preparation of intermediate 1
[0031] 55.56 grams (0.3 mol) of potassium phthalimide and 300 milliliters of acetonitrile were added to a 500 milliliter three-necked flask, and 24.45 grams (0.15 mol) of chloroethylsulfonyl chloride were added dropwise at room temperature. Keeping the reaction temperature not exceeding 60°C, the dropwise addition was completed within 1 hour, after reacting at room temperature for 10 hours, suction filtration, and vacuum drying at 60°C for 10 hours to obtain 51.42 grams of white solid intermediate 1 with a yield of 89.27%; melting point : 239.1-239.7 ℃; IR (KBr pellet, cm −1 ):2947.2, 2362.8, 2341.5, 1793.8, 1782.2, 1745.5, 1705.1, 1462, 1440.8, 1388.7,1363.6, 1259.5, 1217.1, 1178.5, 1103.2,1035.7, 974, 864.1, 850.6, 713.6,563.2, 516.2, 482.2; 1 H NMR (400 MHz, DMSO-D 6 ) δ(ppm): 7.91-7.80(m, 4H), 7.79-7.74(m, 4H), 4.11(s, 4H); HRMS (ESI) m / z 385.0517 (M+H) + .
[0032] (2) Preparation of 2-aminoethylsulfonamide hydrochloride
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com