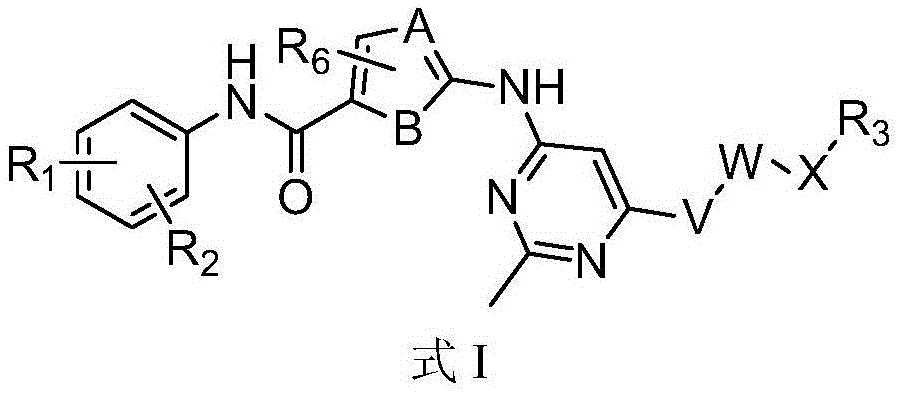
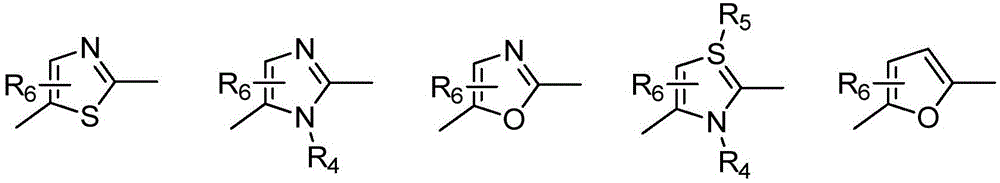
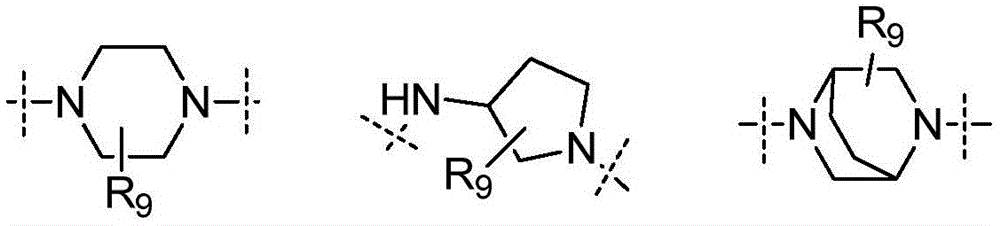
Tyrosine kinase inhibitor and preparation method and use thereof
A technology of tyrosine kinase and inhibitor, applied in the field of tyrosine kinase inhibitor and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Compound 8a, methyl 4-(6-((5-((2-chloro-6-methylphenyl)carbamoyl)-2-thiazolyl)amino)-2-methyl-4-pyrimidinyl) - Preparation of 1-piperazine carboxylate.
[0105] (1) Compound I-7 was synthesized according to the above route.
[0106] Wherein compound I-3, I-4, I-5, I-6 are known compounds, and synthetic method is operated according to existing literature (F.Fernández et al., Synthesis 2001, No.2,239-242; Bang-Chi Chen . et al ARKIVOC 2010(vi), 32-38).
[0107] The characterization data of compound 1-3 are: 1 H NMR (400MHz, CDCl 3 )δppm7.60(d, J=12.10Hz, 1H), 5.32(d, J=12.10Hz, 1H), 3.88(q, J=7.10Hz, 2H), 1.20(t, J=7.10Hz, 3H) .
[0108] The characterization data of compound I-4 are: 1 H NMR (400Hz, DMSO-d 6 )δppm9.28(s,1H),7.45(d,J=12.4Hz,1H),7.27-7.37(d,J=7.5Hz,1H),7.10-7.27(m,J=7.5Hz,2H), 5.58(d, J=12.4Hz, 1H), 3.94(q, J=7Hz, 2H), 2.15(s, 3H), 1.26(t, J=7Hz, 3H).
[0109] The characterization data of compound I-5 are: 1 H NMR (400Hz, DMSO-d 6)δppm 9.63 (s, 1...
Embodiment 2
[0119] Compound 8b, ethyl 4-(6-((5-((2-chloro-6-methylphenyl)carbamoyl)-2-thiazolyl)amino)-2-methyl-4-pyrimidinyl) - Preparation of 1-piperazine carboxylate.
[0120] The preparation method is the same as in Example 1, using compound I-7 to react with ethyl chloroformate. A yellow solid 8b was obtained in 76% yield.
[0121] The characterization data of the compound 8b are: 1 H NMR (400MHz, DMSO-d 6 )δ10.03(s, 1H), 8.31(s, 1H), 7.39(d, J=6.8Hz, 1H), 7.26(d, J=9.3Hz, 2H), 6.21(s, 1H), 4.07( dd,J=13.6,6.6Hz,2H),3.49(s,8H),2.48(s,3H),2.24(s,3H),1.20(t,J=6.9Hz,3H); 13 C NMR (125MHz, DMSO-d 6 )δ163.64,162.51,161.29,160.25,159.57,156.99,156.49,154.70,138.80,133.40,132.44,129.07,128.27,127.04,83.69,61.02,44.02,42.15,24.14,18.32,14.59.ESI-MS m / z: 516.2(M+H) + ,514.1(M-H) - .
[0122] The structural formula of the compound 8b is:
[0123]
Embodiment 3
[0125] Compound 8c, isopropyl 4-(6-((5-((2-chloro-6-methylphenyl)carbamoyl)-2-thiazolyl)amino)-2-methyl-4-pyrimidinyl )-1-piperazine carboxylate preparation.
[0126] The preparation method is the same as in Example 1, using compound I-7 and isopropyl chloroformate. 8c was obtained as a yellow solid in 93% yield.
[0127] The characterization data of the compound 8c are: 1 H NMR (400MHz, DMSO-d 6 )δ9.99(s,1H),8.28(s,1H),7.40(d,J=6.6Hz,1H),7.27(d,J=9.6Hz,2H),6.18(s,1H),4.94- 4.66(m,1H),3.59(s,4H),3.48(s,4H),2.47(s,3H),2.24(s,3H),1.21(d,J=5.7Hz,6H); 13 C NMR (125MHz, DMSO-d 6 )δ163.61,160.32,159.32,158.19,156.49,154.26,138.74,137.83,134.37,133.37,132.38,128.99,128.18,126.97,83.45,68.23,43.95,42.52,24.15,21.95,18.27.ESI-MSm / z:530.2 (M+H) + ,528.2(M-H) - .
[0128] The structural formula of the compound 8c is:
[0129]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com