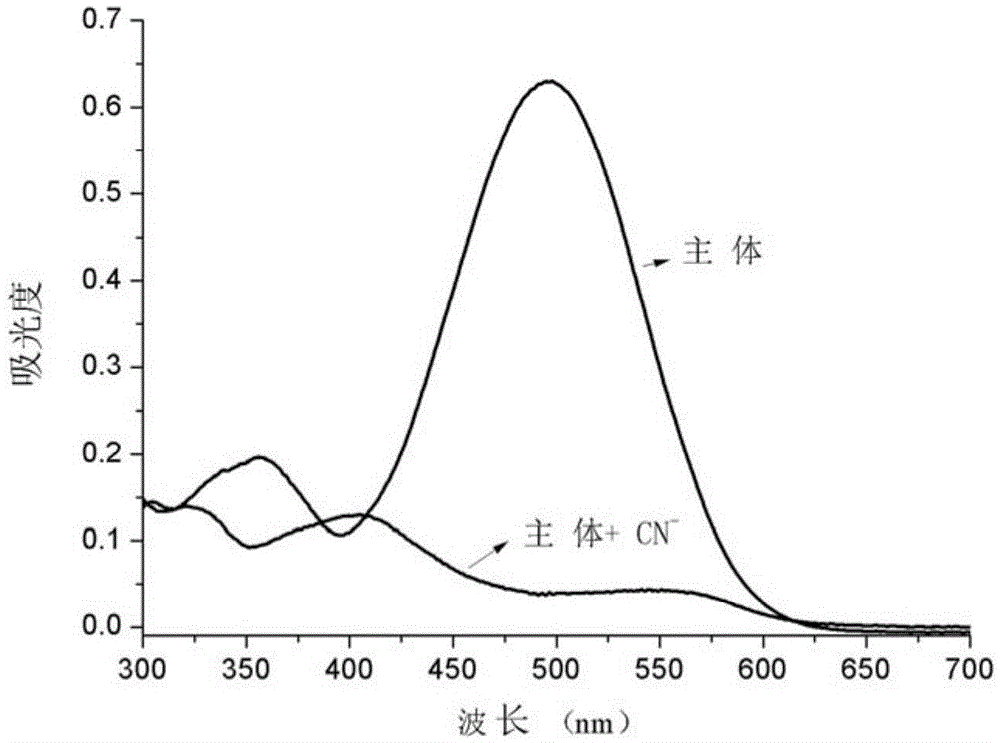
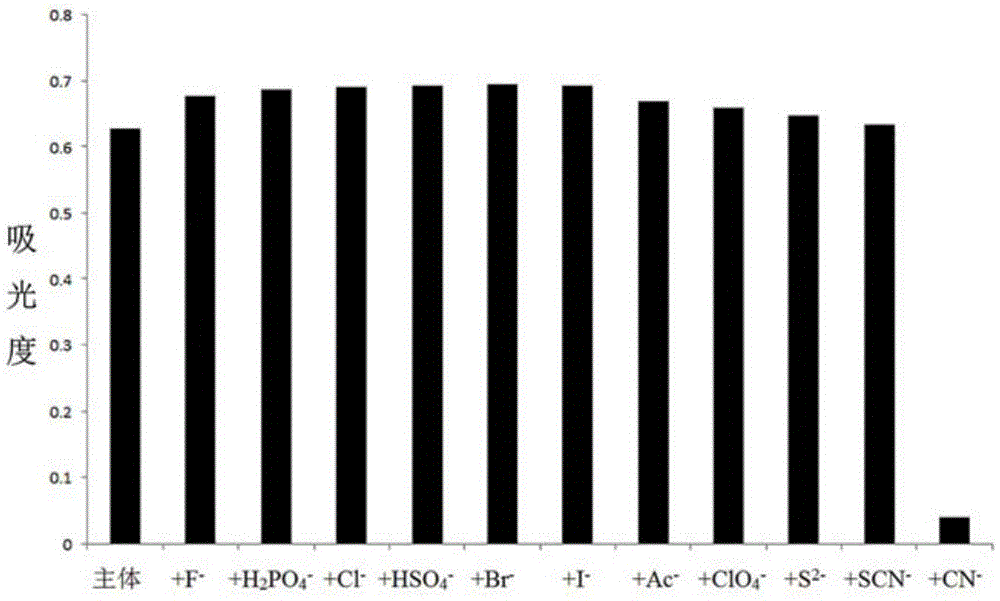
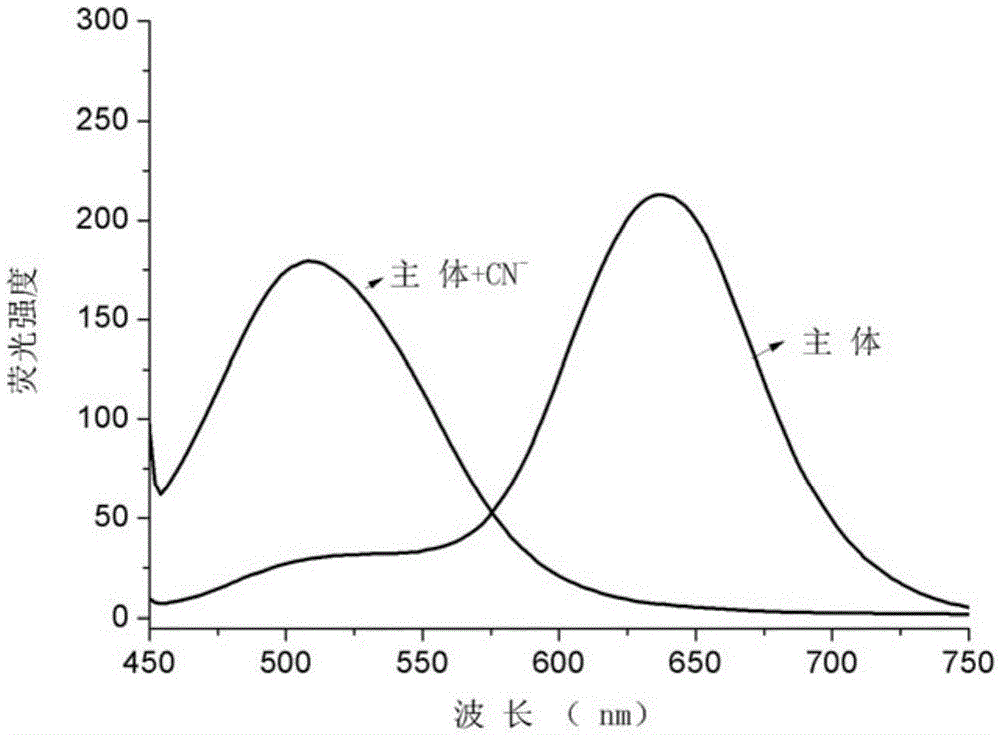
Iodide-N-ethyl-2-(2-H-naphthopyran-3-vinyl) benzothiazole and preparation method and application thereof
A technology of methylbenzothiazole and naphthopyran, which is applied in the field of anion detection, can solve the problems of low fluorescence emission peak, complex receptor structure, and restricted application, and achieves easy-to-obtain raw materials, great application value, and simple synthesis method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0032] 2. CN - Preparation and detection of ion detection test paper
[0033] Based on the cyanide ion detection test paper of iodide-N-ethyl-2-(2-H-naphthopyran-3-vinyl)benzothiazole, the specific preparation method is as follows:
[0034] First, use 0.1~0.5mol L of filter paper -1 Soak in dilute hydrochloric acid for 0.5 to 1 hour, and wash with distilled water until the filtrate is neutral; remove water by suction filtration, and place the filter paper in a vacuum drying box to dry; then iodide the acceptor compound-N-ethyl- 2-(2-H-Naphthopyran-3-vinyl)benzothiazole was dissolved in HEPES-DMSO-H 2 O system (DMSO:H 2 O=1 / 1 (v / v), HEPES: 0.008~0.012M, pH=7.2~7.4), the concentration is 1.0~2.0×10 -3 mol·L -1 The solution was then added dropwise to the treated filter paper to make the HEPES-DMSO-H of the acceptor compound 2 The O solution system is uniformly adsorbed on the filter paper; then the filter paper is dried in a vacuum drying box, and finally cut into 2cm × 2cm...
Example Embodiment
[0039] Example 1
[0040] 2-H-Naphthopyran-3-carbaldehyde (2 mmol) and quaternary ammonium salt of methylbenzothiazole (2.2 mmol) were mixed in absolute ethanol (20 mL), piperidine (0.04 mmol) was added, and the mixture was stirred and refluxed After 6h, the reaction was cooled and suction filtered to obtain a dark green solid, which was rinsed with absolute ethanol for several times to obtain the product iodide-N-ethyl-2-(2-H-naphthopyran-3-vinyl) benzothiazole.
[0041] Yield: Yield 78%, m.p.261-263°C; 1 H NMR(500MHz, DMSO)δ8.45(d,J=8.0Hz,1H),8.34(s,1H),8.28(d,J=8.5Hz,1H),8.20(d,J=8.5Hz,1H) ), 8.09(d, J=15.6Hz, 1H), 7.98(d, J=8.8Hz, 1H), 7.93(d, J=8.1Hz, 1H), 7.88(t, J=7.5Hz, 1H), 7.79(t,J=7.6Hz,1H),7.66(t,J=7.6Hz,1H),7.48(t,J=7.5Hz,1H),7.36(d,J=15.6Hz,1H),7.24( d, J=8.9Hz, 1H), 5.40 (s, 2H), 4.92 (q, J=7.1Hz, 2H), 1.06 (t, J=7.0Hz, 3H). 13 C NMR(126MHz,DMSO)δ171.45,155.04,146.52,141.51,133.93,133.39,130.56,130.02,129.58,128.75,128.52,128.46,125.19,124.93,122.00,117.86,...
Example Embodiment
[0042] Example 2
[0043] 2-H-Naphthopyran-3-carbaldehyde (2 mmol) and quaternary ammonium salt of methylbenzothiazole (2.0 mmol) were mixed in absolute ethanol (20 mL), piperidine (0.04 mmol) was added, and the mixture was stirred and refluxed After 6h, the reaction was cooled and suction filtered to obtain a dark green solid, which was rinsed with absolute ethanol for several times to obtain the product iodide-N-ethyl-2-(2-H-naphthopyran-3-vinyl) benzothiazole.
[0044] Yield: 78%. The characterization data of the synthesized product are the same as those in Example 1.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap