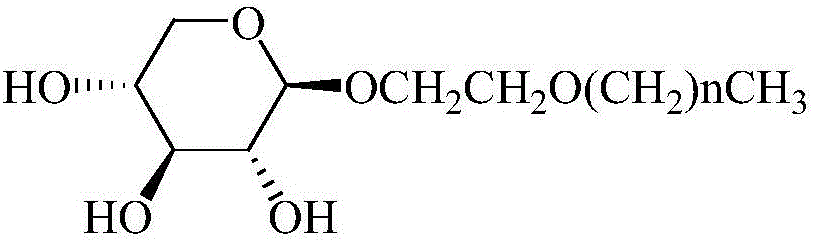Novel xylosidesurfactant
An active agent, xyloside technology, applied in the field of fine chemical surfactants, can solve the problems of limiting the application field of surfactants, reducing water solubility, limiting surface activity, etc., and achieving high application value, enhanced water solubility, and improved water solubility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
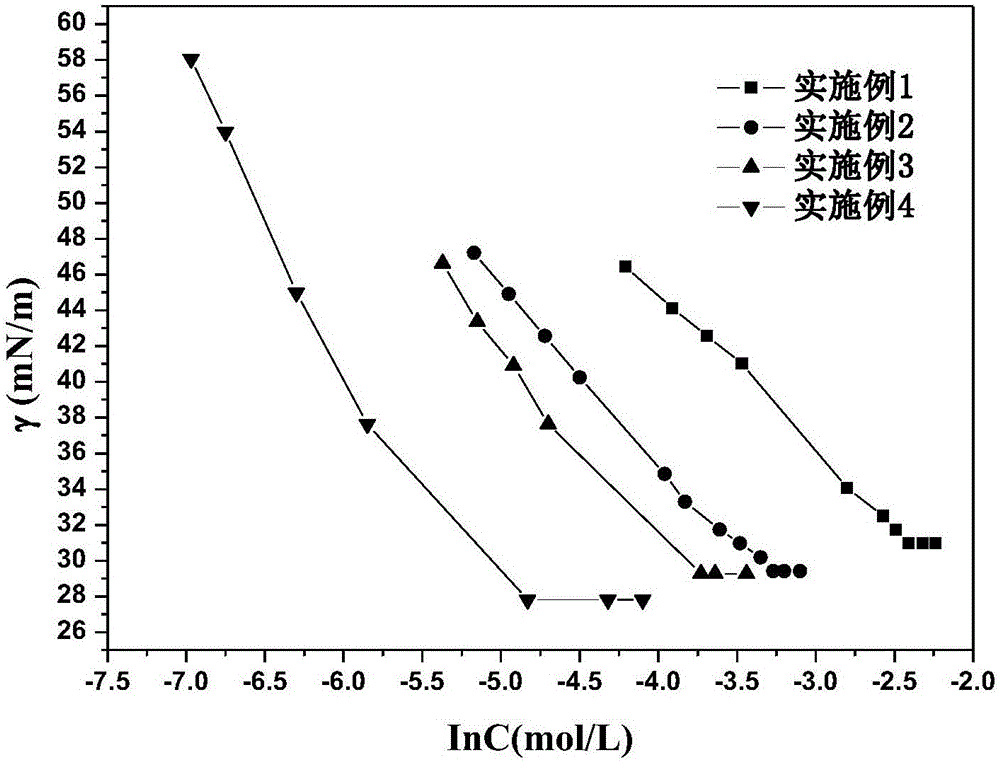
[0030] Example 1: Hexyloxyethyl-β-D-xylopyranoside
[0031] This embodiment provides a preparation method of hexyloxyethyl-β-D-xylopyranoside surfactant, which includes the following steps:
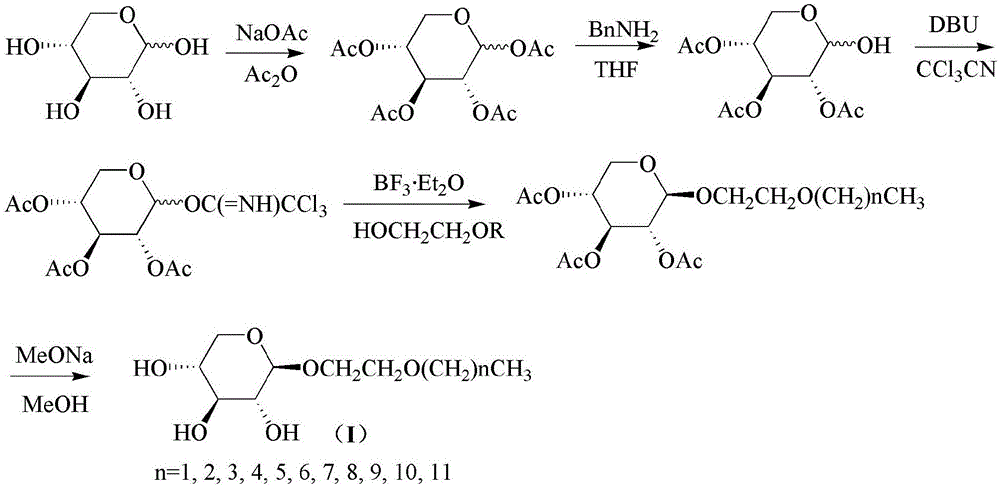
[0032] (1) Add 0.33 mol of dried D-xylose, 1.67 mol of acetic anhydride, and 97.53 mmol of anhydrous sodium acetate to a 500 mL three-necked flask successively, install a reflux condenser, mechanical stirring, and electric heating mantle to heat up the solid After dissolving, remove the heating device and continue stirring until all the solids are dissolved and become clear, then cool to room temperature. Then add 68.63mmol of anhydrous sodium acetate, move the device into the oil bath, heat to reflux for 1h, TLC (developer: V Petroleum ether : V Ethyl acetate =1:1) Monitor the completion of the reaction. Pour the reaction solution into 800mL ice water and stir while it is hot. A large amount of solids are precipitated out. The filter cake is washed with distilled water several times to obta...
Example Embodiment
[0039] Example 2: Heptoxyethyl-β-D-xylopyranoside
[0040] (1) Same as step (1) in Example 1.
[0041] (2) Same as step (2) in Example 1.
[0042] (3) Same as step (3) in Example 1.
[0043] (4) Into a 250mL round-bottomed flask, sequentially add 23.8mmol of 2,3,4-tri-O-acetyl-D-xylopyranosyl trichloroacetimide obtained in step (3), 50mL dichloro Methane Molecular sieve dry product) and 35.6mmmol ethylene glycol monoheptyl ether, stir to dissolve, cool to 0℃ in an ice-water bath, drop 23.8mmol boron trifluoride ether solution, stir for 3h, TLC (developer: V Petroleum ether : V Ethyl acetate =5:1) The reaction is monitored to be complete. The mixed solution was washed successively with saturated sodium bicarbonate aqueous solution and saturated salt solution, dried with anhydrous sodium sulfate, filtered, the filtrate was concentrated, and the column chromatography (V Petroleum ether : V Ethyl acetate =10:1) After separation, 6.20 g of heptyloxyethyl-2,3,4-tri-O-acetyl-β-D-xylopy...
Example Embodiment
[0047] Example 3: Octyloxyethyl-β-D-xylopyranoside
[0048] (1) Same as step (1) in Example 1.
[0049] (2) Same as step (2) in Example 1.
[0050] (3) Same as step (3) in Example 1.
[0051] (4) Into a 250mL round-bottomed flask, sequentially add 23.8mmol of 2,3,4-tri-O-acetyl-D-xylopyranosyl trichloroacetimide obtained in step (3), 50mL dichloro Methane Molecular sieve dry product) and 35.6mmmol ethylene glycol monooctyl ether, stir to dissolve, cool to 0℃ in an ice-water bath, drop 23.8mmol boron trifluoride ether solution, stir for 3h, TLC (developer: V Petroleum ether : V Ethyl acetate =5:1) The reaction is monitored to be complete. The mixed solution was washed successively with saturated sodium bicarbonate aqueous solution and saturated salt solution, dried with anhydrous sodium sulfate, filtered, the filtrate was concentrated, and the column chromatography (V Petroleum ether : V Ethyl acetate =10:1) After separation, 6.75 g of octoxyethyl-2,3,4-tri-O-acetyl-β-D-xylopyran...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com