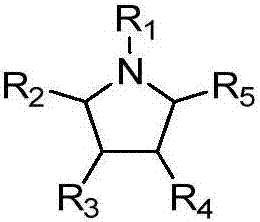
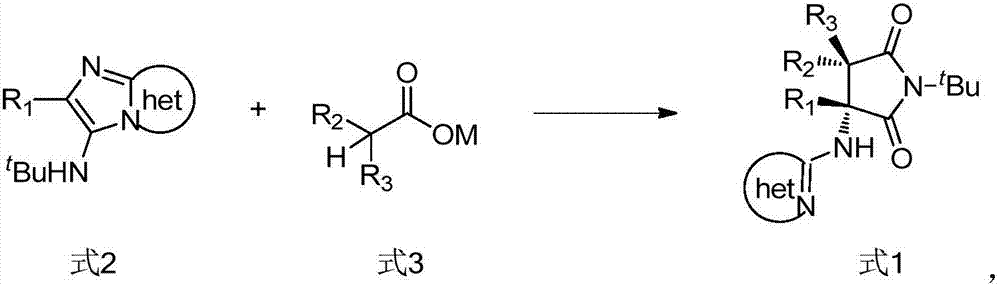
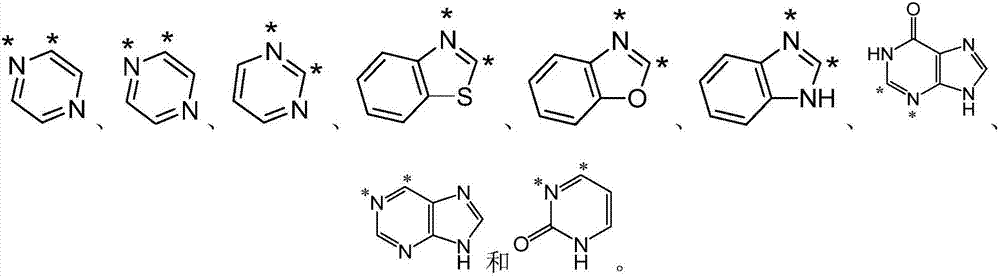
Preparation method of pyrrolidine-2, 5-dione derivatives
A technology of alkyl and compound, applied in the field of medicine, can solve problems to be developed and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] In this example, the influence of the type of reaction solvent, the combination of silver salt, the reaction temperature and the amount of aliphatic silver salt was studied.
[0113] Carry out the reaction according to the following reaction formula, wherein the whole reaction system is carried out under nitrogen atmosphere, the concentration of substrate 5a is 0.1mmol, the concentration of aliphatic silver salt is 0.22mmol, the volume of reaction solvent is 10mL, and the reaction time is 24 hours.
[0114]
[0115] The influence of the combination of table 1 reaction solvent type, silver salt, temperature of reaction and the amount of aliphatic silver salt
[0116] sequence silver source temperature(°C) solvent 6a yield (%) 1 a
AgOAc 120 toluene 50 2 b
AgOAc 120 toluene 80 3 c
AgOAc 120 toluene 80 4 b
AgOAc 120 DMF 72 5 b
AgOAc 120 wxya 70 6 b
AgOAc 120 1,2-DCB 70 7 b ...
Embodiment 2
[0120] In this example, the study substrate R 1 The effect of the type of group.
[0121] The reaction was carried out according to the following reaction formula, wherein the concentration of substrate 5 was 0.1 mmol, the concentration of AgOAc was 0.22 mmol, the volume of toluene was 10 mL, the reaction temperature was 120° C., and the time was 24 hours.
[0122]
[0123] It can be seen that the electronic properties of the substituents on the aromatic ring do not affect the transformation, such as N-Fused imidazoles with electroneutral (6a) and halogen atom groups (6b), electron donating groups (6c and 6d) and absorbing Electron groups (6e and 6f) also succeeded in giving the desired products in moderate to good yields (71-80%). In addition, substituents on the aromatic ring system also gave the desired results (6g, 75%; 6h, 80%; 6i, 65%; 6j, 68%). N-Fused imidazole with 2-furyl (6k), N-methyl-2-pyrrolyl (6l), 2-thiazolyl (6m), 3-indolyl (6n) and ethyl carboxylate ( 6...
Embodiment 3
[0125] In this example, the effect of heterocycles in the substrate was investigated.
[0126] The reaction was carried out according to the following reaction formula, wherein the concentration of substrate 7 was 0.1 mmol, the concentration of AgOAc was 0.22 mmol, the volume of toluene was 10 mL, the reaction temperature was 120° C., and the time was 24 hours.
[0127]
[0128] Heterocyclic compounds pyrazine, pyrimidine, benzothiazole and adenine were used instead of the pyridine ring (the pyridine ring of substrate 5 in Example 1) as substrates. found regardless of R 1 Regardless of group differences, pyrazine, pyrimidine, benzothiazole, and adenine as heterocyclic substituents reacted smoothly, and the products were obtained in good to excellent yields (8a, 78%; 8b, 60%; 8c, 65%; 8d, 70%, 8e, 50%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com