Aromatic hydrocarbon ruthenium complex and preparation method and applications thereof
A technology of ruthenium complexes and aromatic hydrocarbons, applied in the directions of ruthenium organic compounds, platinum group organic compounds, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
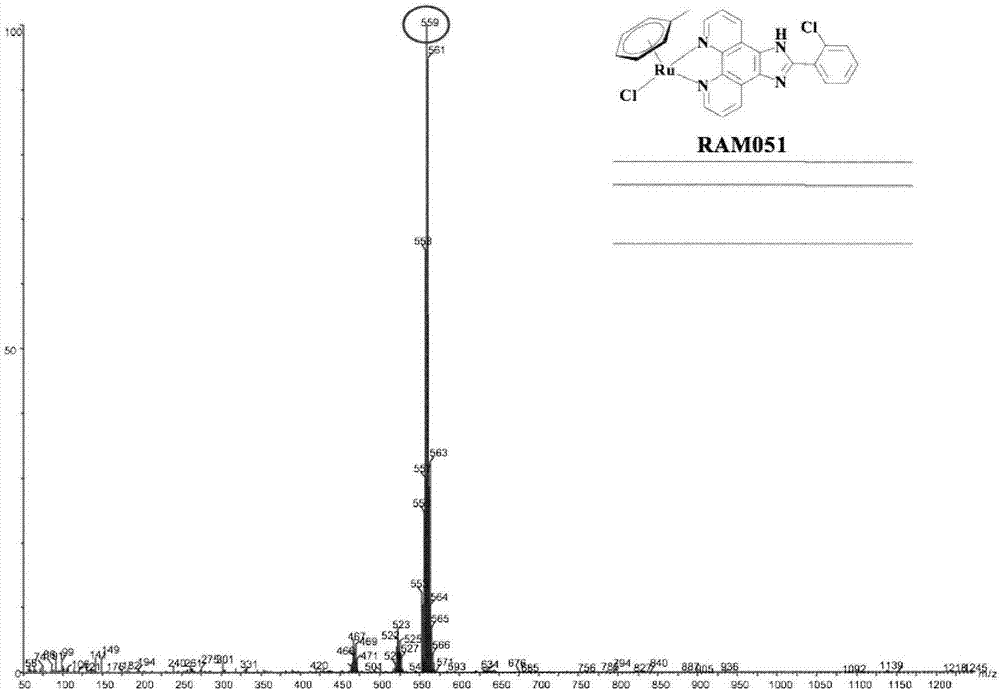
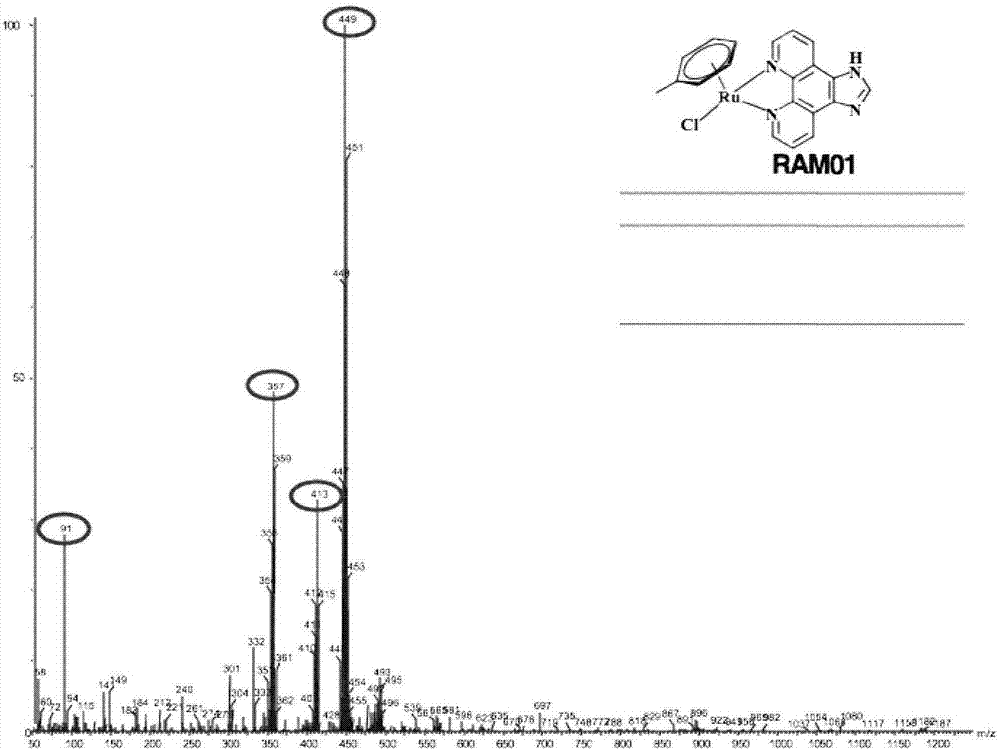
[0060] Microwave Synthesis of RAM01
[0061] by RuCl 3 Reaction with 1-methyl-1,4-cyclohexadiene at 30-180°C, microwave irradiation for 15s-60min to obtain the intermediate, the intermediate obtained by the above reaction and imidazol[4,5f][1,10]phenanthrene Roline (IP) was irradiated with microwave for 15s at 60°C in dichloromethane solvent. After the reaction, it was dissolved with a small amount of methanol to obtain RAM01, whose structural formula is as follows.
[0062]
Embodiment 2
[0064] Microwave Synthesis of RAM09
[0065] The foregoing steps are the same as in Example 1;
[0066] The intermediate and 2-phenyl-imidazo[4,5f][1,10]phenanthroline (PIP) were irradiated with microwaves at 120°C for 30 minutes in dichloromethane solvent to obtain RAM09 with the following structural formula.
[0067]
Embodiment 3
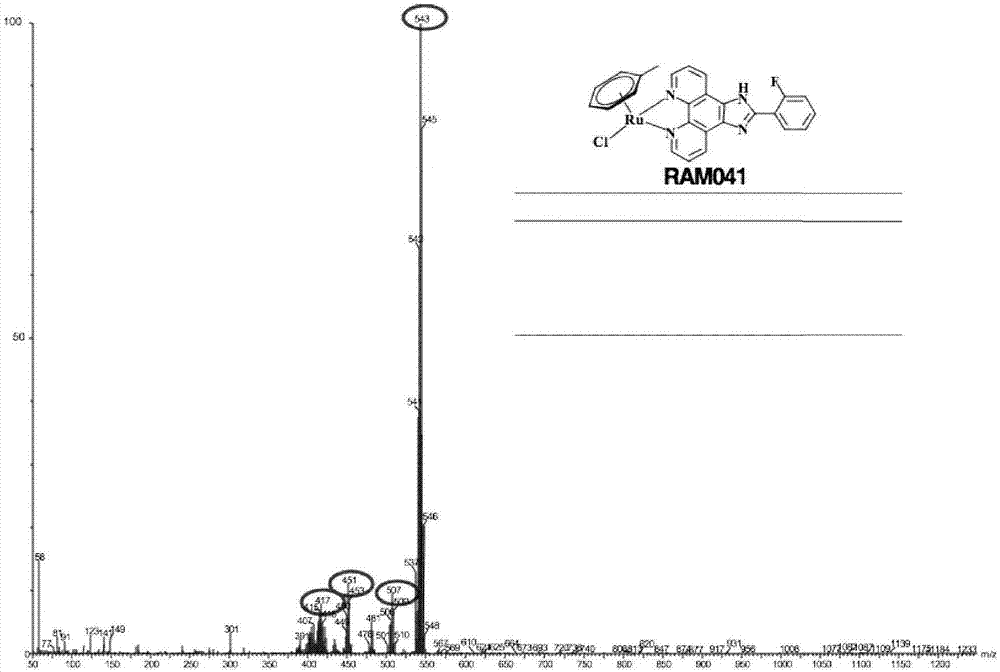
[0069] Microwave synthesis of RAM031
[0070] The foregoing steps are the same as in Example 1;
[0071] The intermediate and 2-(2-fluorophenyl)-imidazol[4,5f][1,10]phenanthroline (PIP) were irradiated by microwave at 90°C for 30min in dichloromethane solvent.
[0072]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com