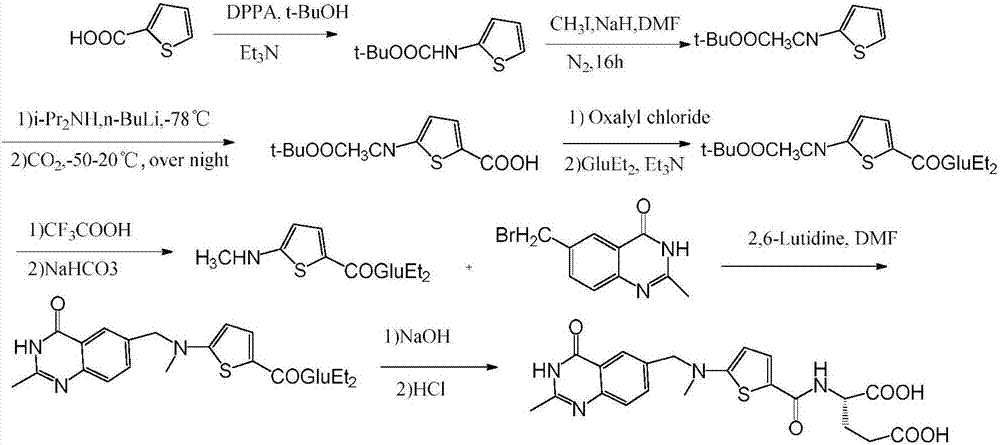
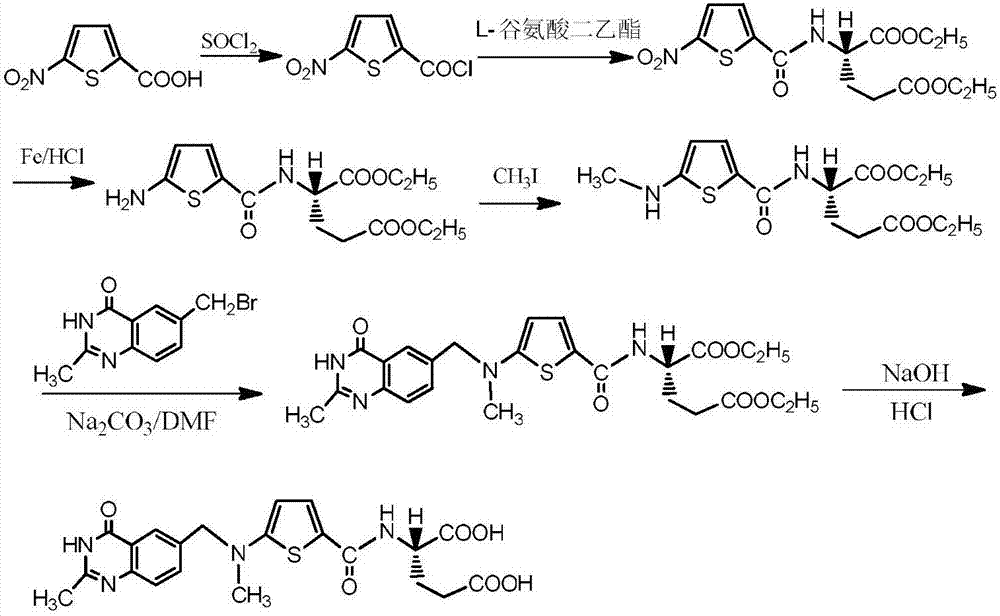
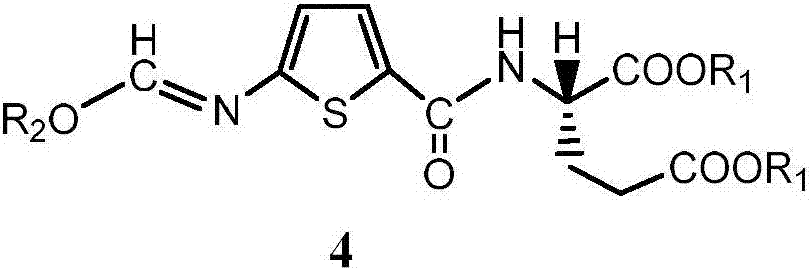
5-((alkoxy methylene) amino) thienyl-2-formyl group)-L-glutamic acid dialkyl ester and preparation method thereof
A methyl and alkyl technology, applied in the field of drug synthesis, can solve the problems of many by-products, long reaction time, inconvenient post-processing, etc., and achieve the effects of fast reaction, shortened reaction time, convenient post-processing and storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of compound 5 (R 1 for ethyl)
[0042]
[0043] 5-Nitrothiophene-2-formyl-L-glutamic acid diethyl ester (2.2g) was dissolved in methanol (5.2g), and added dropwise with iron powder (2.1g), methanol (5.2g), concentrated In the mixed solution of hydrochloric acid (2.8g), the temperature is controlled not to exceed 50°C. After the dripping is completed, the temperature is raised to 70-80°C for reaction. TLC monitors that the compound is completely reacted, filtered with suction, and concentrated under reduced pressure below 80°C. 20 g of toluene was divided into two times to take out the solvent, added ethyl acetate (9.9 g) to dissolve, suction filtered, added drinking water (11.0 g) to the filtrate, washed with 20% aqueous sodium carbonate solution (11.0 g), adjusted the pH to 8-9, The organic phase was obtained, and the organic phase was washed with saturated brine (8.8 g), dried over anhydrous sodium sulfate, and suction filtered, and t...
Embodiment 2
[0044] Embodiment 2: the preparation of compound 4a (R 2 for methyl)
[0045]
[0046]Compound 5 (13g) and trimethyl orthoformate (78g) were heated and dissolved, heated to 80°C, and reacted for 2 hours. TLC monitored that the reaction was complete, cooled to below 50°C, concentrated under reduced pressure to remove trimethyl orthoformate, and obtained The solid was 13.8g, the yield was 93%, and the HPLC purity was 99.7%. 1 H NMR (500MHz, CDCl 3 ):δ8.39(s,1H),8.01(d,J=10.0Hz,1H),7.58(dd,J=12.5Hz,1H),7.50(d,J=5.0Hz,1H),4.51(m ,1H),4.21(m,2H),4.13(m,2H),3.40(s,3H),2.35(dd,J=7.5Hz,2H),2.29(d,J=10.0Hz,2H),1.29 (s,3H),1.23(s,3H); MS(ES) m / z371.42[M+H] + .
Embodiment 3
[0047] Embodiment 3: the preparation of compound 4b (R 2 for ethyl)
[0048]
[0049] Compound 5 (13g) and triethyl orthoformate (78g) were heated and dissolved, heated to 90°C, and reacted for 2 hours. TLC monitored that the reaction was complete, cooled to below 50°C, concentrated under reduced pressure to remove triethyl orthoformate, and obtained Solid (14.2 g, yield 93%, HPLC purity 99.4%). 1 H NMR (500MHz, CDCl 3 ):δ8.36(s,1H),8.01(d,J=10.0Hz,1H),7.57(dd,J=12.5Hz,1H),7.50(d,J=5.0Hz,1H),4.52(m ,1H),4.37(m,2H),4.21(m,2H),4.13(m,2H),3.43(s,3H),2.35(dd,J=7.5Hz,2H),2.29(d,J= 10.0Hz,2H),1.29(s,3H),1.22(s,3H); MS(ES) m / z 385.32[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com