Chinese herbal medicine capsule preparation for treating adenomyosis and preparation method thereof
A technology for adenomyosis and Chinese herbal medicine, which is applied in the directions of capsule delivery, pharmaceutical formulations, and medical preparations containing active ingredients, etc., can solve the problems of non-universal application, obvious side effects, and large differences in the efficacy of adenomyosis, and achieves convenient use. , Affirmative effect of curative effect, overcoming the effect of large difference in curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
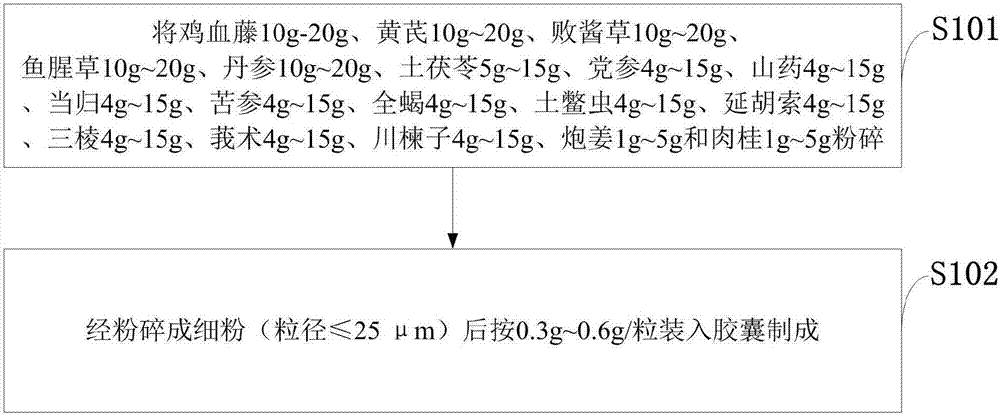
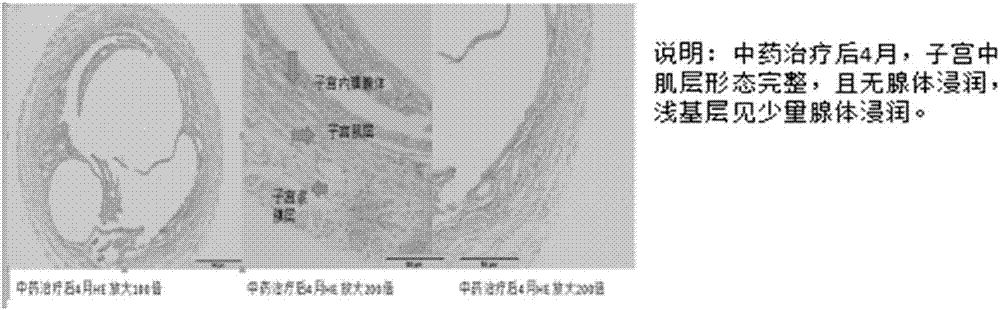
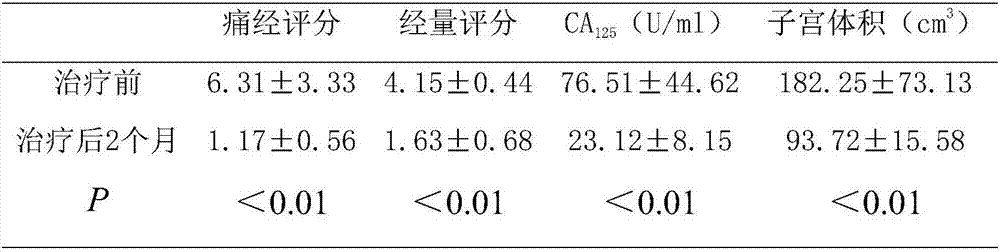
[0018] Such as figure 1 As shown, the preparation method of the Chinese herbal medicine capsule preparation for the treatment of adenomyosis provided by the embodiments of the present invention may further comprise the steps:
[0019] S101: Spatholobus 10g-20g, Astragalus 10g-20g, Patrinia 10g-20g, Houttuynia cordata 10g-20g, Salvia miltiorrhiza 10g-20g, Smilax 5g-15g, Codonopsis 4g-15g, Chinese yam 4g-15g, Angelica 4g~15g, Sophora flavescens 4g~15g, Scorpion 4g~15g, Wood Beetle 4g~15g, Corydalis 4g~15g, Sanleng 4g~15g, Curcuma 4g~15g, Toosendan 4g~15g, Paojiang 1g~ 5g and cinnamon 1g~5g crushed;
[0020] S102: It is prepared by crushing into fine powder (particle size ≤ 25 μm) and then filling it into capsules at a rate of 0.3g to 0.6g / capsule.
[0021] The taking method of the Chinese herbal medicine capsule preparation for treating adenomyosis provided by the embodiment of the invention is: 3 times a day, 3 capsules each time, orally, and 1 month is a course of treatment....
Embodiment 1
[0024] The Chinese herbal medicine capsule preparation for the treatment of adenomyosis provided by the embodiments of the present invention consists of Caulis Spatholobus 15g, Astragalus 15g, Patrinia 15g, Houttuynia cordata 15g, Salvia miltiorrhiza 15g, Smilax cocos 10g, Codonopsis 8g, yam 8g, angelica 8g, Composition of Sophora flavescens 8g, scorpion 7g, ground beetle 7g, Corydalis 8g, Sanleng 5g, Curcuma 5g, Toosendan 5g, Paojiang 3g and cinnamon 2g.
[0025] The preparation method of the Chinese herbal medicine capsule preparation for the treatment of adenomyosis provided by the embodiments of the present invention comprises:
[0026] Spatholobus 15g, Astragalus 15g, Patrinia 15g, Houttuynia cordata 15g, Salvia miltiorrhiza 15g, Smilax tuckahoe 10g, Codonopsis 8g, Chinese yam 8g, angelica 8g, flavescens 8g, scorpion 7g, wood beetle 7g, Corydalis 8g, Sanleng 5g, Curcuma 5g, Toosendan 5g, Paojiang 3g and Cinnamon 2g are crushed into fine powder (particle size ≤ 25μm) and t...
Embodiment 2
[0028] The Chinese herbal medicine capsule preparation for the treatment of adenomyosis provided by the embodiments of the present invention consists of Caulis Spatholobus 10g, Astragalus 20g, Patrinia 10g, Houttuynia cordata 20g, Salvia miltiorrhiza 18g, Smilax 10g, Codonopsis 4g, Chinese yam 5g, angelica 8g, Composition of Sophora flavescens 9g, scorpion 6g, wood beetle 7g, Corydalis 10g, Sanleng 15g, Curcuma 4g, Toosendan 9g, Paojiang 2g and cinnamon 2g.
[0029] The preparation method of the Chinese herbal medicine capsule preparation for the treatment of adenomyosis provided by the embodiments of the present invention comprises the following steps:
[0030] Step 1, take Spatholobus 10g, Astragalus 20g, Patrinia 10g, Houttuynia cordata 20g, Salvia miltiorrhiza 18g, Smilax miltiorrhiza 10g, Codonopsis 4g, yam 5g, angelica 8g, flavescens 9g, scorpion 6g, ground beetle 7g, Corydalis 10g, Sanleng 15g, Curcuma 4g, Toosendan 9g, Paojiang 2g and Cinnamon 2g crushed;
[0031] Ste...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com