Orlistat liposome and its preparation method and application in antitumor drugs
An orlistat and liposome technology, applied in the field of biomedicine, can solve problems such as orlistat liposomes that are not used for treatment and/or anti-tumor, achieve good medicinal value, increase solubility, reduce stimulating effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
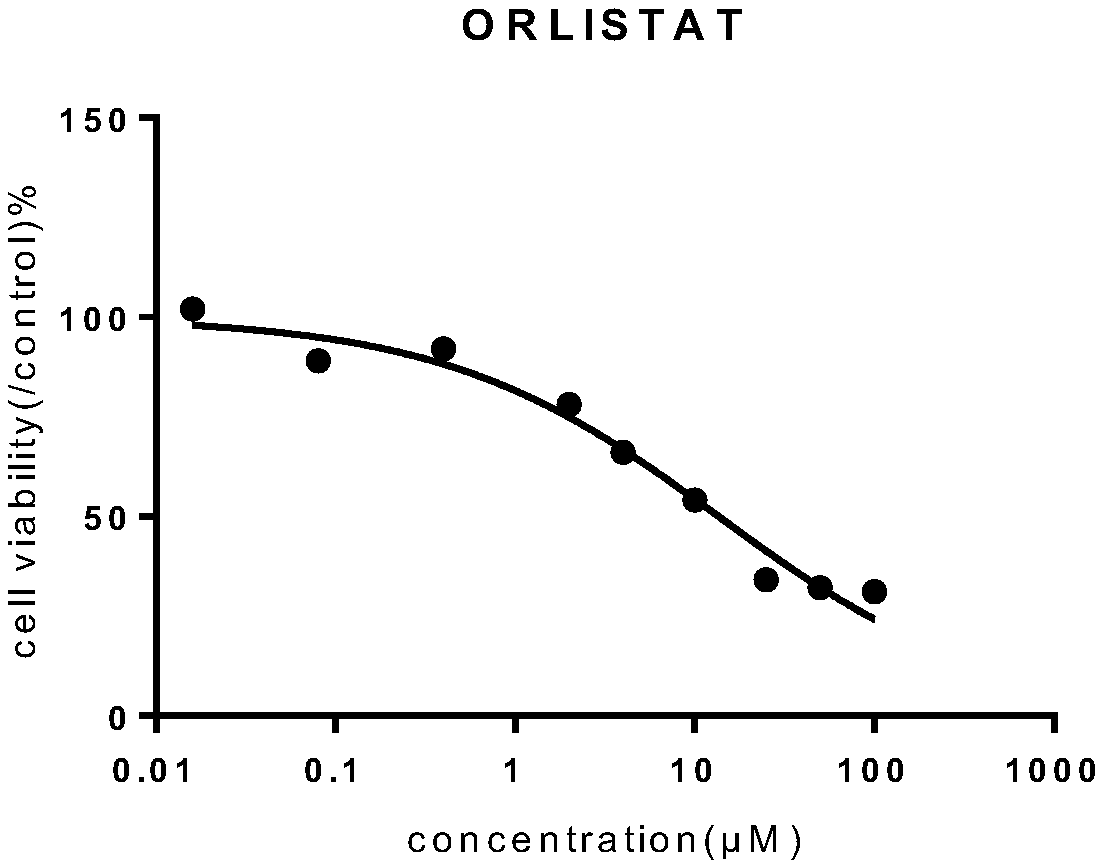
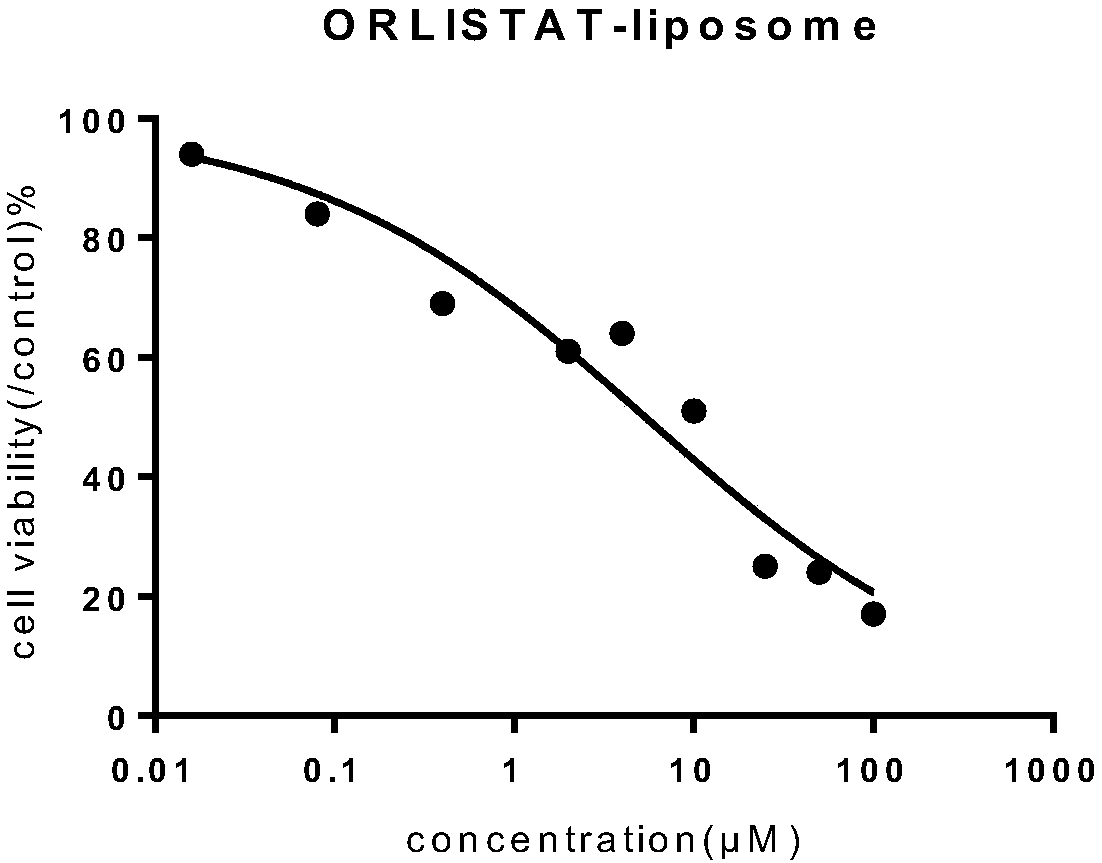
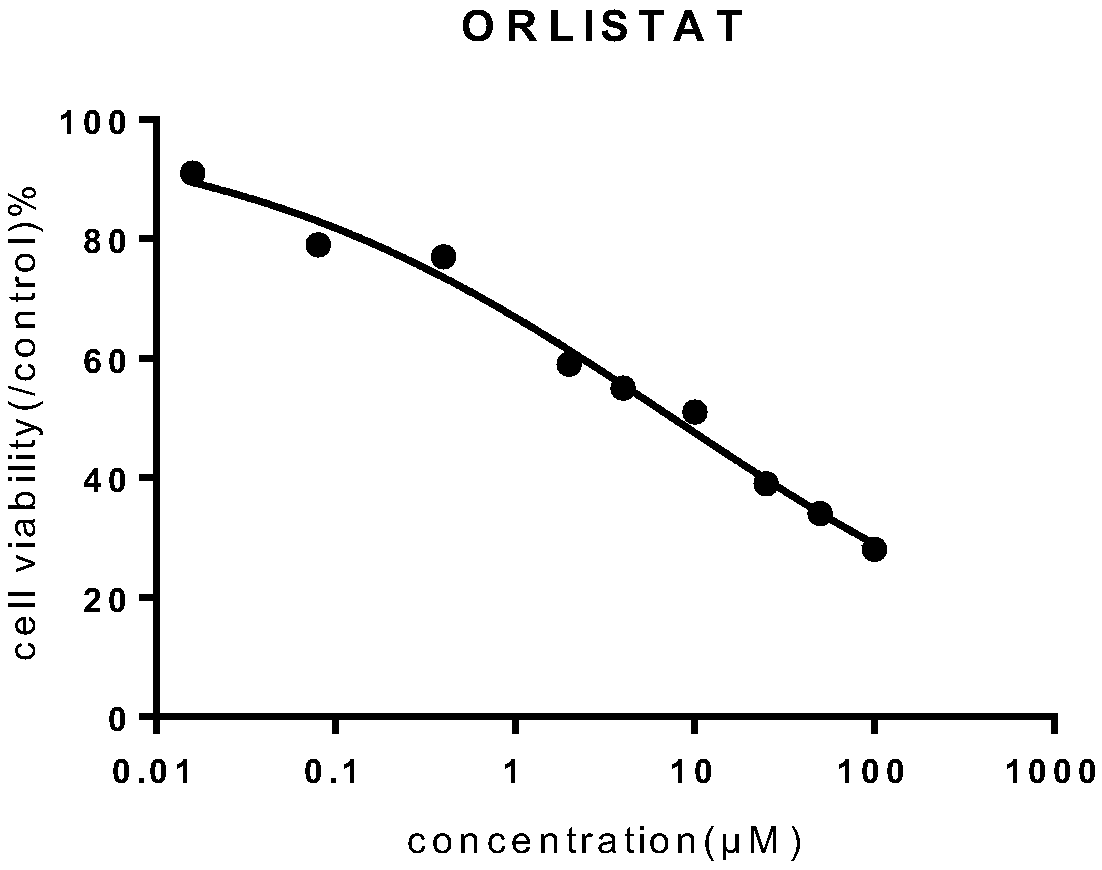
[0032] Weigh soybean lecithin 25mg, distearoylphosphatidylcholine 5mg, mPEG 2000 - 2.5mg of DPPE, 6mg of cholesterol and 4mg of orlistat were uniformly mixed; adding 15ml of absolute ethanol and stirring to dissolve, ultrasonication for 2min to mix evenly, slowly inject the obtained solution into 20ml of PBS buffer, stir at 60°C, The ethanol was removed by pressure, an appropriate amount of PBS was added, and the orlistat liposome L1 was obtained by repeatedly extruding through a 200 nm membrane with a liposome extruder.
Embodiment 2
[0034] Weigh soybean lecithin 24mg, distearoylphosphatidylcholine 4mg, mPEG 2000 - 2mg of DPPE, 7mg of cholesterol and 4mg of orlistat are uniformly mixed; add 12ml of absolute ethanol and stir to dissolve, ultrasonication for 2min to mix evenly, slowly inject the obtained solution into 18ml of PBS buffer, stir at 60°C, and depressurize The ethanol was removed, and an appropriate amount of PBS was added, and the orlistat liposome L2 was obtained by repeatedly extruding through a 200 nm membrane with a liposome extruder.
Embodiment 3
[0036] Weigh soybean lecithin 24mg, distearoylphosphatidylcholine 6mg, mPEG 2000 - 2 mg of DPPE, 5 mg of cholesterol and 5 mg of orlistat are uniformly mixed; add 12 ml of absolute ethanol and stir to dissolve, ultrasonically mix for 2 minutes, slowly inject the obtained solution into 18 ml of PBS buffer, stir at 60°C, and depressurize The ethanol was removed, and an appropriate amount of PBS was added, and the orlistat liposome L3 was obtained by repeatedly extruding through a 200 nm membrane with a liposome extruder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com