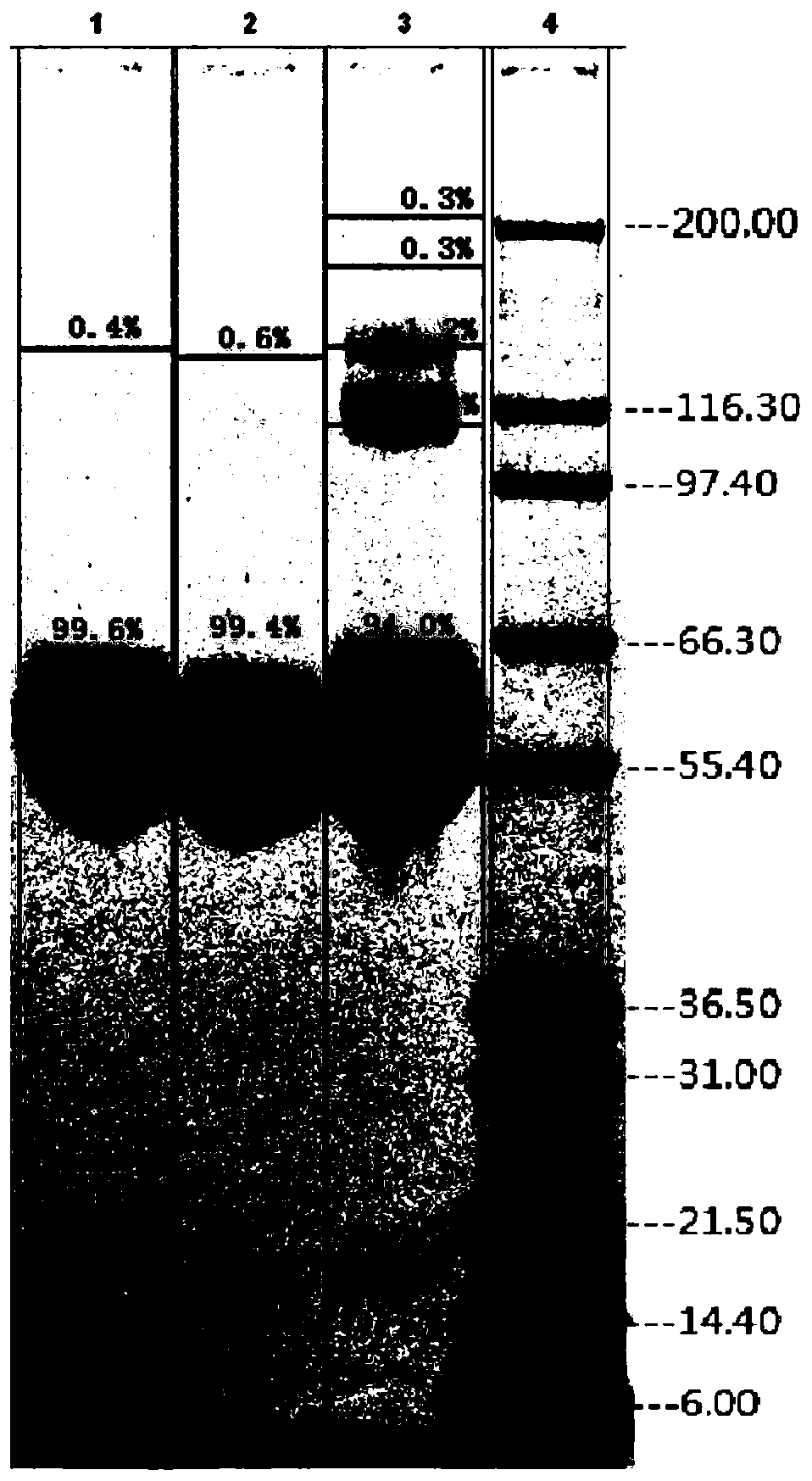
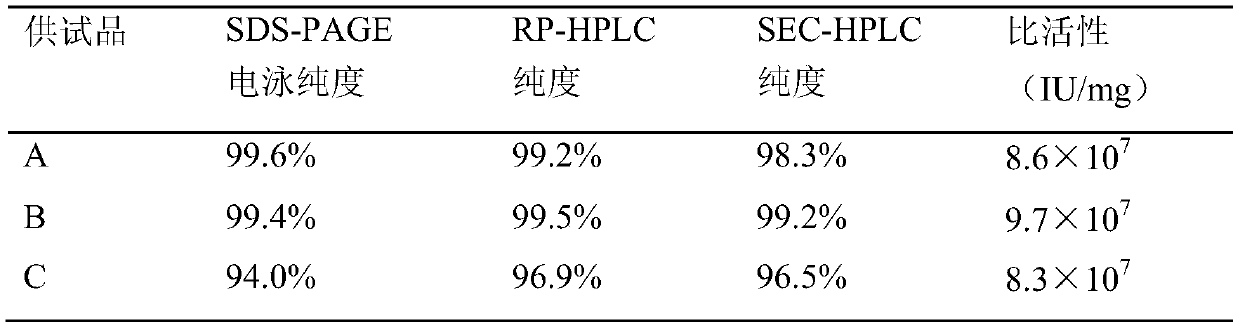
A purification method of pegylated recombinant human granulocyte stimulating factor
A technology of PEGylation and stimulatory factors, applied in peptide preparation methods, chemical instruments and methods, organic chemistry, etc., can solve the problem of low purity of PEG-G-CSF, which can only reach 90% or 95%. and other problems, to achieve the effect of short purification cycle, easy operation and easy process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Step 1, PEG-G-CSF modified product buffer replacement
[0044] Equilibrate Sephadex G-25 chromatography column with 20mmol / L acetic acid-sodium acetate, pH4.0 buffer solution, flow rate 150cm / h, equilibrate 2 times column volume;
[0045] Load the PEG-G-CSF modified product clarified by the cellulose acetate filter membrane, the single loading volume shall not exceed 1 / 4 of the column volume, and the flow rate shall be 150cm / h;
[0046] Elute with 20 mmol / L acetic acid-sodium acetate, pH 4.0 buffer, collect the sample peaks, and obtain the crude PEG-G-CSF.
[0047] Step 2, SP Sepharose HP column chromatography purification of PEG-G-CSF crude product
[0048] Equilibrate the SP Sepharose HP chromatography column with 20mmol / L acetic acid-sodium acetate, pH4.0 buffer solution, flow rate 80cm / h, equilibrate 5 times the column volume, until the UV and conductivity are stable;
[0049] Load the PEG-G-CSF crude product at a flow rate of 50cm / h;
[0050] After loading the s...
Embodiment 2
[0058] Step 1, PEG-G-CSF modified product buffer replacement
[0059] The PEG-G-CSF modified product was clarified and filtered through a 0.45 μm filter element (CVHL71TP3) to obtain a clarified reaction solution;
[0060] 20mmol / L acetic acid-sodium acetate, pH4.0 buffer balance hollow fiber column UFP-10-E-55 and hollow fiber system FlexStand;
[0061] Pump the clarified reaction solution into the storage tank of the FlexStand hollow fiber system, start the system, adjust the return valve, and control the transmembrane pressure (TMP) at 20-30PSI;
[0062] Use 20mmol / L acetic acid-sodium acetate, pH4.0 buffer as the replacement solution, and replace with a constant volume, so that the rate of the replacement solution added is the same as that of the permeate, and the volume of the sample is kept constant in the hollow fiber system storage tank. The replacement ends when the dosage is about 5 times the volume of the sample;
[0063] Samples were collected from the bottom val...
Embodiment 3
[0075] Step 1, PEG-G-CSF modified product buffer replacement
[0076] Same as embodiment two: step 1
[0077] Step 2, SP Sepharose HP column chromatography purification of PEG-G-CSF crude product
[0078] Operate according to the purification conditions in CN101172161A Example 3, first wash 5 times the volume with 0.5M NaOH solution, then wash with purified water to neutrality, then balance the SP Sepharose HP layer with 20mmol / L acetic acid-sodium acetate, pH4.0 buffer solution Analysis column, flow rate 120cm / h, equilibrate 5 times the column volume, until the UV and conductivity are stable;
[0079] Load the crude PEG-G-CSF at a flow rate of 120cm / h;
[0080] After the sample loading is completed, wash the chromatography column with 20mmol / L acetic acid-sodium acetate, pH4.0 buffer solution for 10 times the column volume, and the flow rate is 120cm / h;
[0081] Use 20mmol / L acetic acid-sodium acetate, pH4.0 solution as solution A, and 20mmol / L acetic acid-sodium acetate, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com