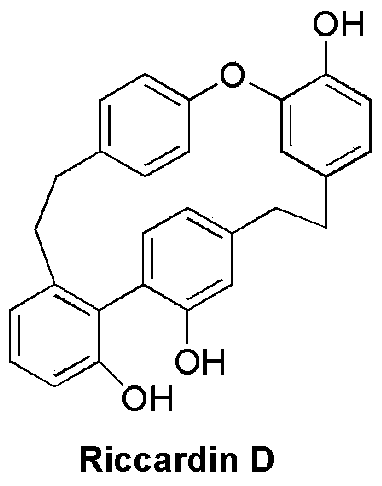
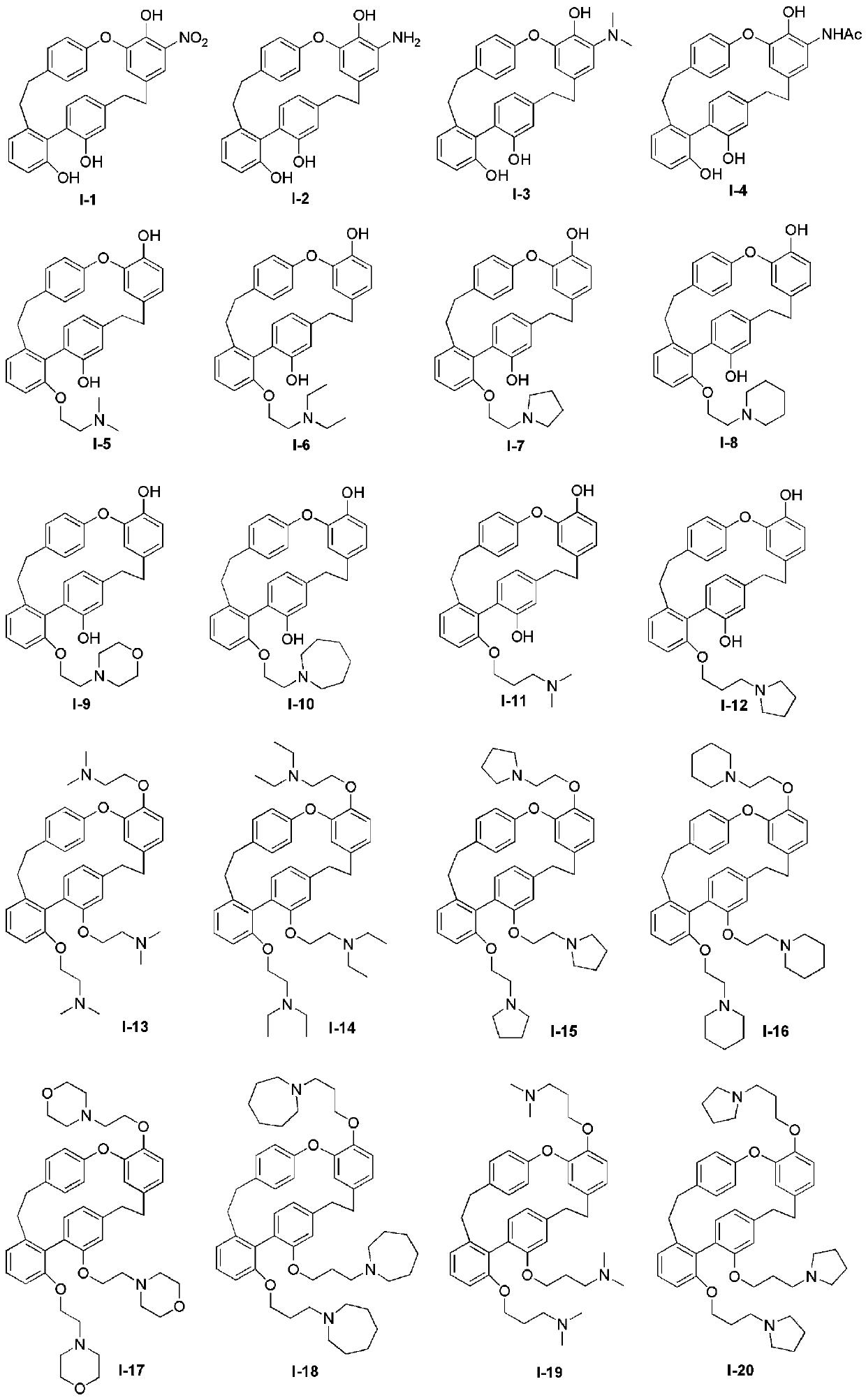
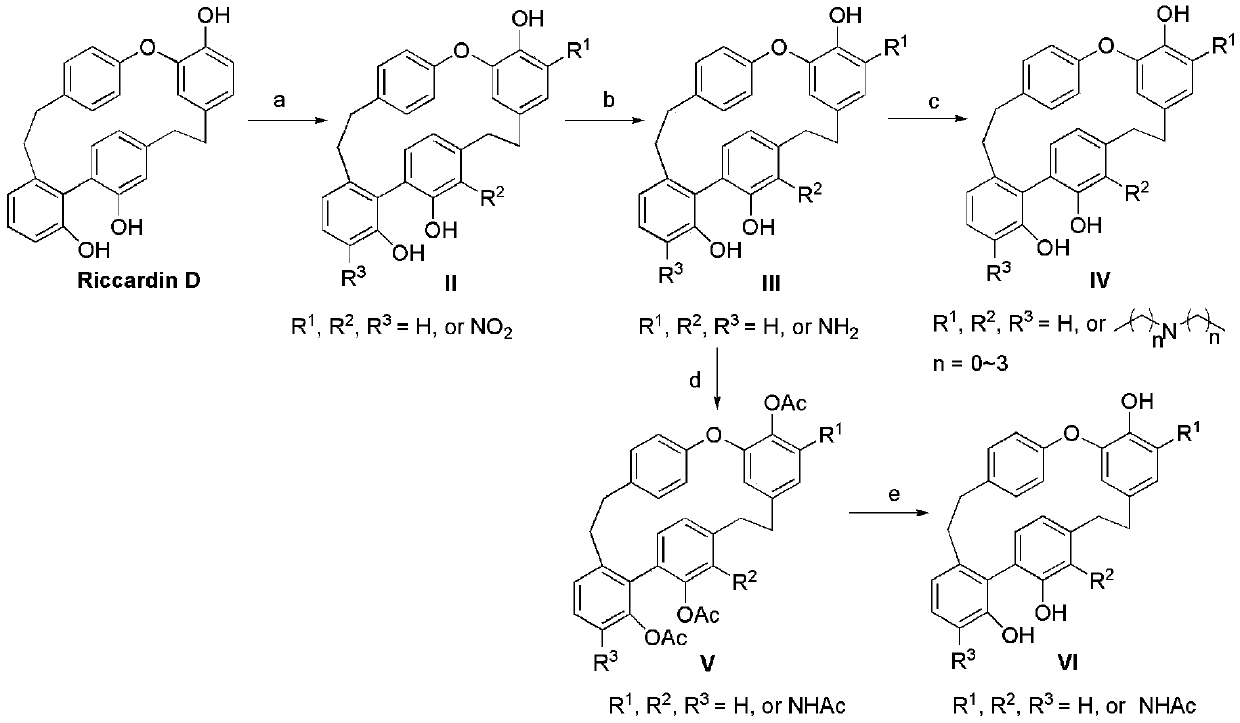
Nitrogen-containing derivatives of lamellarin d, their preparation methods and their use in the treatment of tumor diseases
A technology for tumors and drugs, applied in the field of nitrogen-containing derivatives of orcin D and its preparation, can solve the problems of increased lysosomal membrane penetration, cell apoptosis, etc., and achieve improved selectivity, high tumor selectivity, and targeting strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1: the preparation of compound I-1 to I-4
[0068]
[0069] Preparation of Compound I-1
[0070] A solution of nitric acid (5.3 μL) in acetic acid (0.5 mL) was slowly added dropwise to dichloromethane (0.7 mL) of phyllonin D (50 mg) at 0°C. Stir at room temperature for 24 hours. After the reaction, pour the reaction system into ice water, extract with dichloromethane, combine the organic phases, wash with water three times, dry over sodium sulfate, filter, evaporate the solvent, and purify by silica gel column chromatography to obtain compound I- 1. MS m / z 470(M+1); 1 H NMR (600MHz, CDCl 3 )δ10.72(s,1H),7.63(s,1H),7.38(t, J=7.8Hz,1H),7.28(s,1H),7.10(d,J=7.7Hz,1H),6.96( d,J=8.1Hz,1H),6.93(d,J=8.0Hz,1H),6.88(m,3H),6.84(d,J=8.1Hz,1H),6.56(s,1H),6.38( d,J=7.6Hz,1H),5.81(s,1H),4.96(s,1H),4.94(s,1H),3.06-2.57(m,8H).
[0071] Preparation of Compound I-2
[0072] Dissolve compound I-1 (98mg) in ethyl acetate (5mL), add palladium carbon (5mg), and stir magne...
Embodiment 2
[0077] Embodiment 2: the preparation of compound 1-5
[0078]
[0079] Dissolve phyllonin D (0.5mmol), potassium carbonate (1.5mmol) and 2-chloro-N,N-dimethylethylamine (0.5mmol) in acetone (10mL) solution, heat to reflux, stir for 24 hours, react After completion, filter and remove the solvent, and the obtained product is purified by silica gel column chromatography to obtain the pure compound I-5. MS m / z 496(M+1); 1 H NMR (CDCl 3 ) δ7.35(t, J=7.9Hz, 1H), 7.12(d, J=7.8Hz, 1H), 7.02(d, J=7.9Hz, 1H), 6.96-6.93(m, 2H), 6.89( d,J=7.6Hz,1H),6.86-6.77(m,3H),6.75(dd,J=8.1,1.6Hz,1H),6.65(d,J=7.6Hz,1H), 6.21(s,1H ),5.38(d,J=1.6Hz,1H),4.17-4.02(m,2H),3.07-2.96(m,2H),2.96-2.80(m,4H),2.70-2.61(m,1H), 2.61-2.53 (m, 2H), 2.42 (t, J=12.0Hz, 1H), 2.29 (s, 6H).
Embodiment 3
[0080] Embodiment 3: the preparation of compound 1-6
[0081]
[0082] Dissolve phyllonin D (0.5mmol), potassium carbonate (1.5mmol) and 2-chloro-N,N-diethylethylamine (0.5mmol) in acetone (10mL) solution, heat to reflux, stir for 24 hours, react After completion, filter and remove the solvent, and the obtained product is purified by silica gel column chromatography to obtain the pure compound I-6. MS m / z 524(M+1); 1 H NMR (CDCl 3 ) δ7.38(t, J=7.9Hz, 1H), 7.16(d, J=7.7Hz, 1H), 6.99(d, J=7.3Hz, 1H), 6.93(d, J=7.9Hz, 2H) ,6.94-6.82(m,4H),6.74(d,J=7.5Hz,1H),6.51(d,J=6.1Hz,1H),6.28(s,1H),5.40(s,1H),4.30- 4.20(m, 2H), 2.98-2.90(m, 4H), 2.82(d, J=10.5Hz, 2H), 2.70(s, 4H), 2.63(t, J=11.1Hz, 1H), 2.50(t , J=11.1Hz, 1H), 1.27(s, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com