HER2-resisting antibody-medicine conjugate and applications thereof
A technology of drug conjugates and conjugates, applied in the direction of antibodies, drug combinations, anti-tumor drugs, etc., can solve problems such as difficult separation of antibody-drug conjugate mixtures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0223] The coupling modes for preparing the antibody-drug conjugate of the present invention include two coupling modes, K-Lock and C-Lock. In the K-Lock coupling method, the drug molecule is coupled to the lysine (K) residue in the antibody sequence, and in the C-Lock coupling method, the drug molecule is coupled to the cysteine residue in the antibody sequence (C) The conjugation method of the antibody-drug conjugate of the present invention is outlined below.
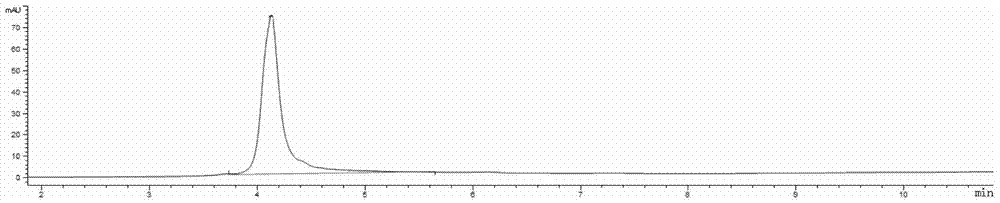
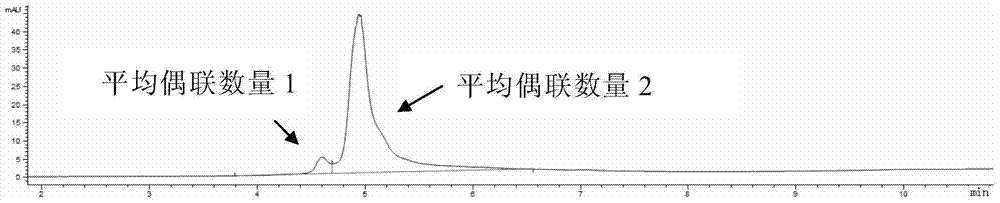
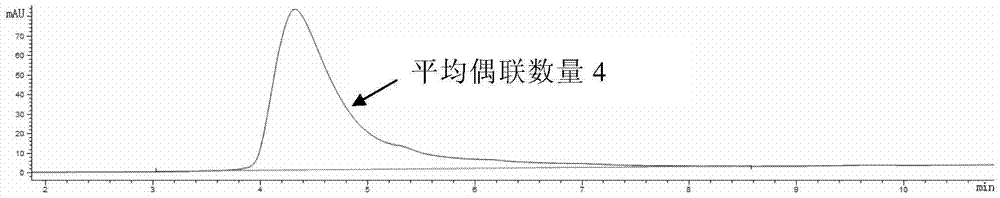
[0224] K-Lock method: Antibodies can be directly linked to L1-D in a mild solution system. For example, use 6-10 times excess L1-D to react with antibody for 3-16 hours at room temperature, and ultra-filter to remove excess small molecule L1-D. The antibody-drug conjugate was loaded onto a hydrophobic chromatography column (HIC), and purified to obtain an anti-HER2 antibody-drug conjugate with a coupling number of 2.
[0225] C-Lock method: After the antibody is reduced by TCEP, it is directly linked to L2-D in a...
Embodiment 1
[0243] The synthesis of embodiment 1 compound 7
[0244]
[0245] Synthesis of compound 3:
[0246] Compound 2 (261 mg, 0.52 mmol) was dissolved in 6 mL of DMF, and HATU (217 mg, 0.57 mmol), DIEA (362 μL, 2.08 mmol), and amino compound 1 (213 mg, 0.52 mmol) were added. Stir for 30 minutes, then add 400 μL of piperidine, and continue stirring for 10 minutes. The reaction solution was concentrated and directly purified by HPLC to obtain compound 3 (171 mg, 60%). MS m / z 548.3 (M+H).
[0247] Compound 5:
[0248] Compound 4 (37 mg, 0.15 mmol) was dissolved in 4 mL of DMF, and HATU (59 mg, 0.15 mmol), DIEA (108 μL, 0.6 mmol), and amino compound 3 (102 mg, 0.15 mmol) were added. The reaction solution was stirred for 30 minutes, evaporated to dryness under reduced pressure, then dissolved in 2 mL of dichloromethane, added with 1 mL of TFA and stirred for 10 minutes. The reaction solution was evaporated to dryness under reduced pressure and purified by HPLC to obtain compound ...
Embodiment 2
[0251] The synthesis of embodiment 2 compound 11
[0252]
[0253] Compound 2 (130 mg, 0.26 mmol) was dissolved in 3 mL of DMF, HATU (110 mg, 0.29 mmol), DIEA (175 μL, 1 mmol), and amino compound 1 (110 mg, 0.27 mmol) were added. The reaction solution continued to stir for 30 minutes, and was rotary evaporated to dryness. The residue was dissolved in TFA / dichloromethane (1 / 4, v / v, 5 mL) and stirred for 30 minutes. The reaction solution was evaporated to dryness under reduced pressure and purified by HPLC to obtain compound 8 (108 mg, 65%). MS m / z 670.5 (M+H).
[0254] Compound 6 (85 mg, 0.12 mmol) was dissolved in 2 mL of DMF, HATU (48 mg, 0.12 mmol), DIEA (83 μL, 0.48 mmol), and amino compound 8 (94 mg, 0.12 mmol) were added. The reaction solution was stirred for 30 minutes, then piperidine (0.2 mL) was added and stirred for 30 minutes. The reaction solution was concentrated and purified by HPLC to obtain compound 9 (87 mg, 63%). MS m / z 1028.7 (M+H).
[0255]To a solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com