Combinations of an anti-HER2 antibody-drug conjugate and chemotherapeutic agents, and methods of use
A chemotherapeutic agent, antibody technology, applied in the direction of drug combination, antibody medical components, chemical instruments and methods, etc., can solve problems such as low efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0275] Example 1 : Preparation of trastuzumab-MCC-DM1
[0276] since Trastuzumab was purified by buffer exchange at 20 mg / mL in 50 mM potassium phosphate / 50 mM sodium chloride / 2 mM EDTA, pH 6.5, with 7.5 to 10 molar equivalents of succinimidyl 4-(N-maleimide Methyl)cyclohexane-1-carboxylate (SMCC, Pierce Biotechnology, Inc), 20 mM in DMSO or DMA (dimethylacetamide), 6.7 mg / mL (US 2005 / 0169933; US 2005 / 0276812) treated . After stirring at ambient temperature under argon for 2-4 hours, the reaction mixture was filtered through a Sephadex G25 column equilibrated with 50 mM potassium phosphate / 50 mM sodium chloride / 2 mM EDTA, pH 6.5. Alternatively, the reaction mixture was gel filtered with pH 6 of 30 mM citrate and 150 mM sodium chloride. Fractions containing antibody were pooled and assayed. The recovery of trastuzumab-SMCC was 88%.
[0277] The drug-linker intermediate trastuzumab-MCC from above was diluted to a final concentration of 10 mg / ml with 50 mM potassium phosph...
Embodiment 2
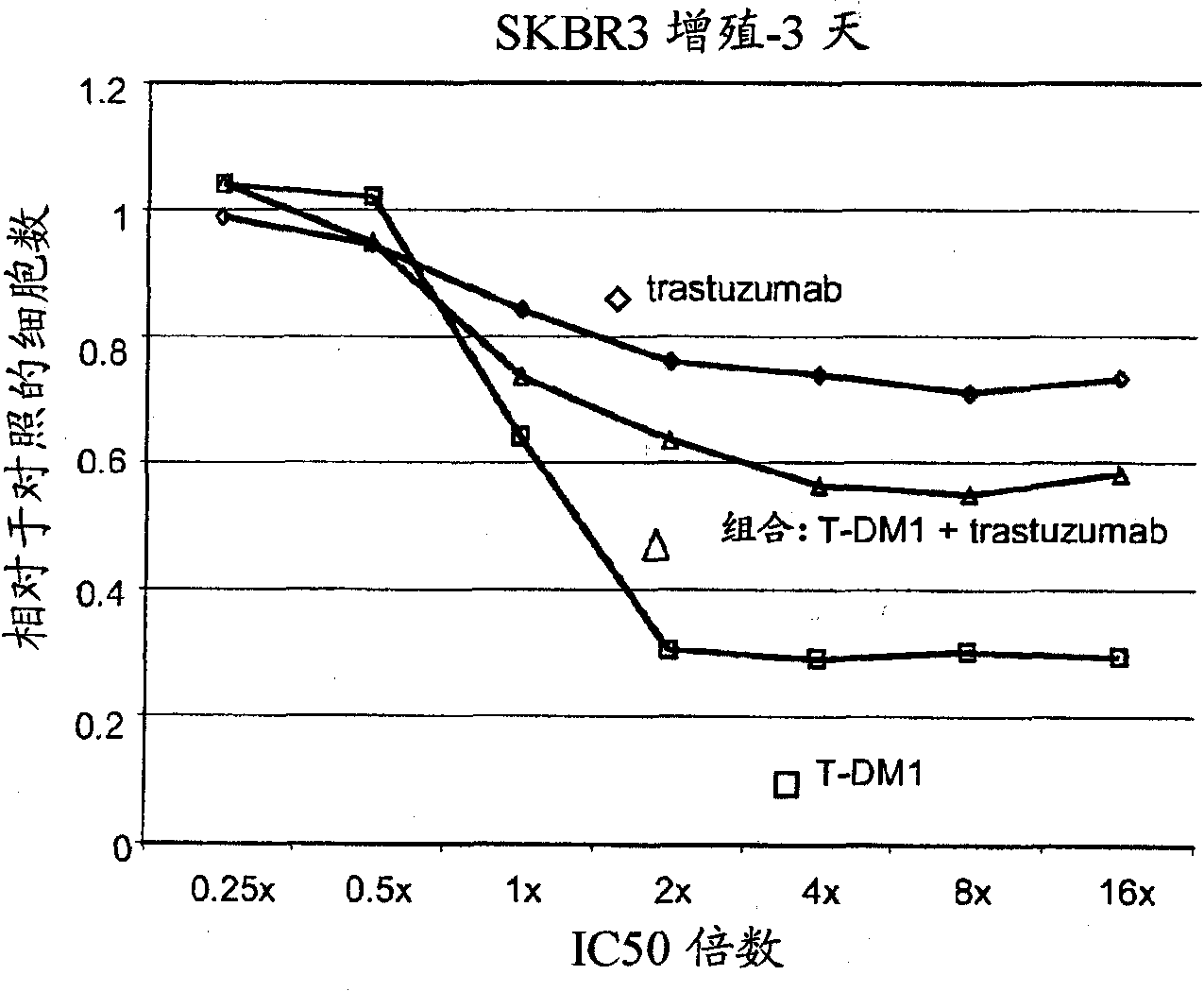
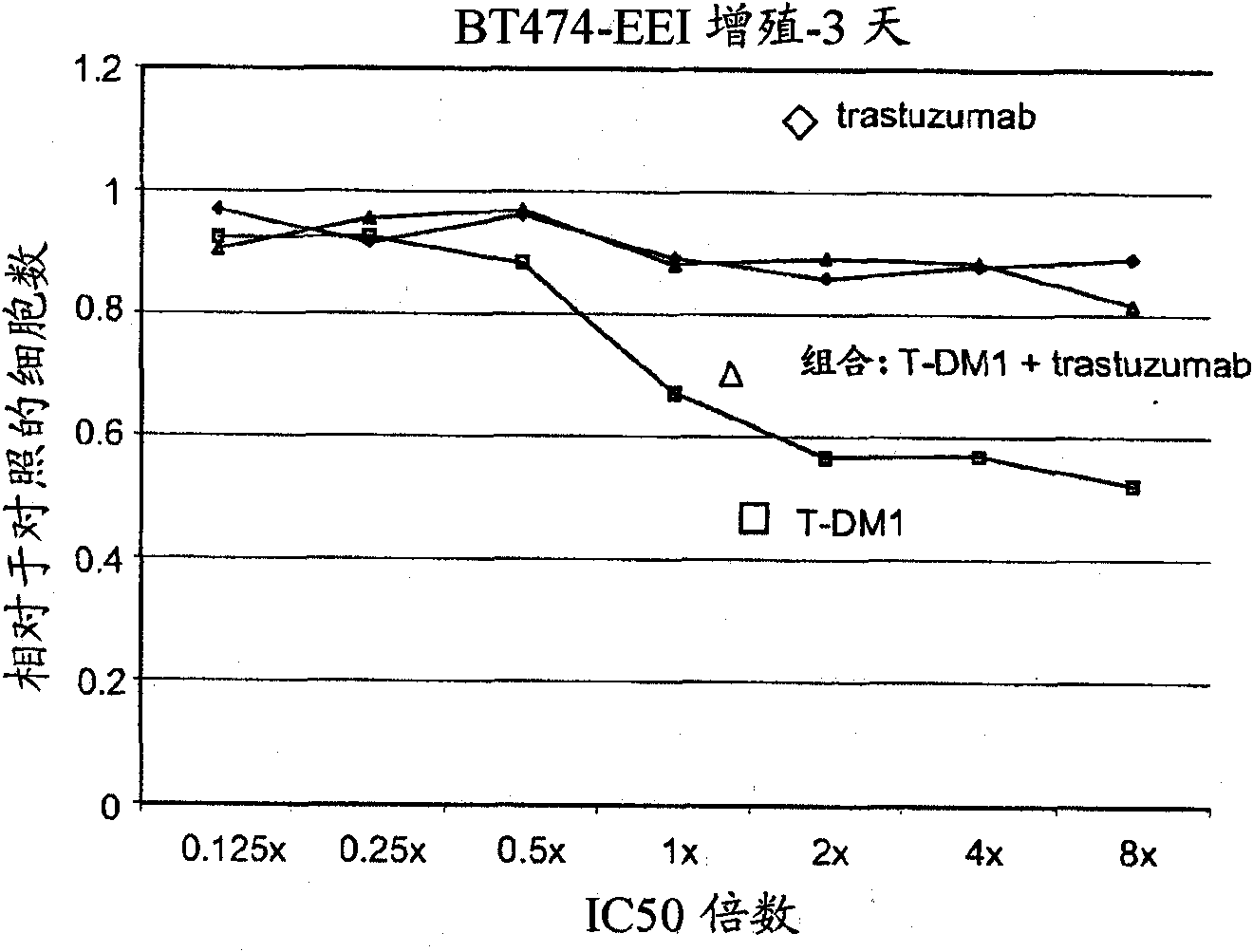
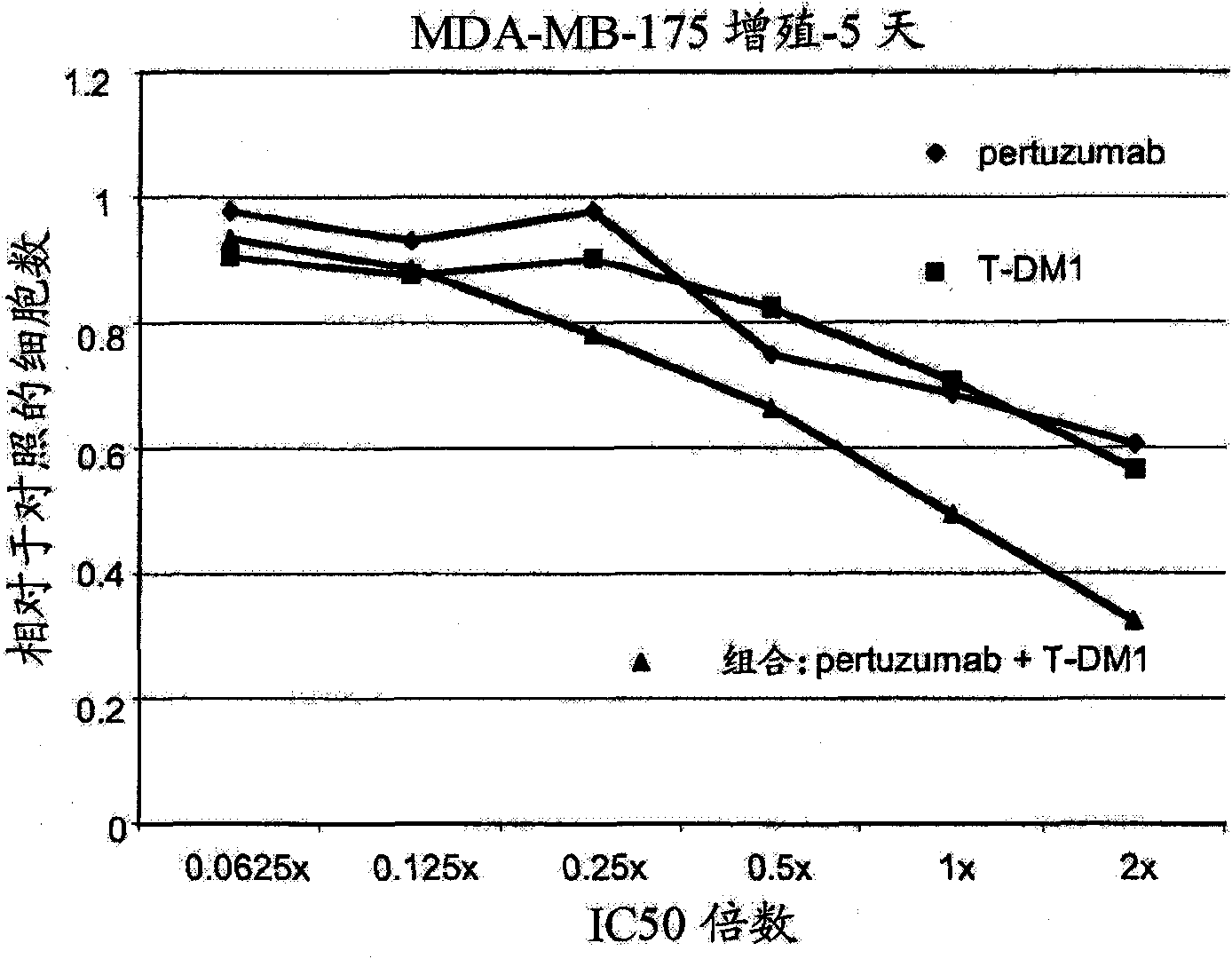
[0281] Example 2 : In Vitro Cell Proliferation Assay
[0282] The efficacy of the combinations of the invention was measured by a cell proliferation assay using the following protocol (Promega Corp. Technical Bulletin TB288; Mendoza et al. (2002) Cancer Res. 62:5485-5488). Cell-Titer Glo assay reagents and protocols are commercially available (Promega). The assay assesses the ability of a compound to enter cells and affect cell proliferation. The assay principle is to determine the number of viable cells present by quantifying cellular ATP. Cell-Titer Glo refers to the reagent used for this quantification. It is a homogeneous assay in which addition of Cell-Titer Glo results in cell lysis and generation of a luminescent signal via a luciferase reaction. The luminescent signal is proportional to the amount of ATP present.
[0283] DMSO and media plates: 96-well conical bottom polypropylene plates (Nunc, catalog # 249946)
[0284] Cell plate: 384-well black with lid, tran...
Embodiment 3
[0303] Example 3 : Tumor xenografts in vitro
[0304] Animals suitable for transgenic experiments are available from standard commercial sources. Groups of female CB-17SCID beige mice (Charles River Laboratory) were implanted with 3 million KPL-4 (Her2-overexpressing) breast cancer cells in the mammary fat pad with Matrigel. Groups of female athymic nude mice (Charles RiverLaboratory or Harlan) were implanted with 2x 2mm of MMTV-Her2Fo5 transgenic mammary tumors in the mammary fat pad 3debris. Mouse xenografts were dosed with drug, drug combination, or vehicle on day 0 according to the schedule specified for each tumor model. 5-FU, gemcitabine, carboplatin, and B20-4.1 administered intraperitoneally, pertuzumab administered intravenously or intraperitoneally as indicated, trastuzumab-MCC-DM1 and docetaxel administered intravenously, lapatinib, GDC-0941, and ABT-869 passed Oral administration by gavage. Tumor dimensions were recorded twice weekly during the study. Mouse ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com