Method of discovering fluoro-containing compounds
A technology of fluorinated compounds and compounds, applied in compound screening, fluorine/hydrogen fluoride, medical preparations containing active ingredients, etc., can solve the problems of a large number of raw materials and the danger of fluorinated reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0154] Example 1: Electrochemical fluorination of rosiglitazone
[0155]
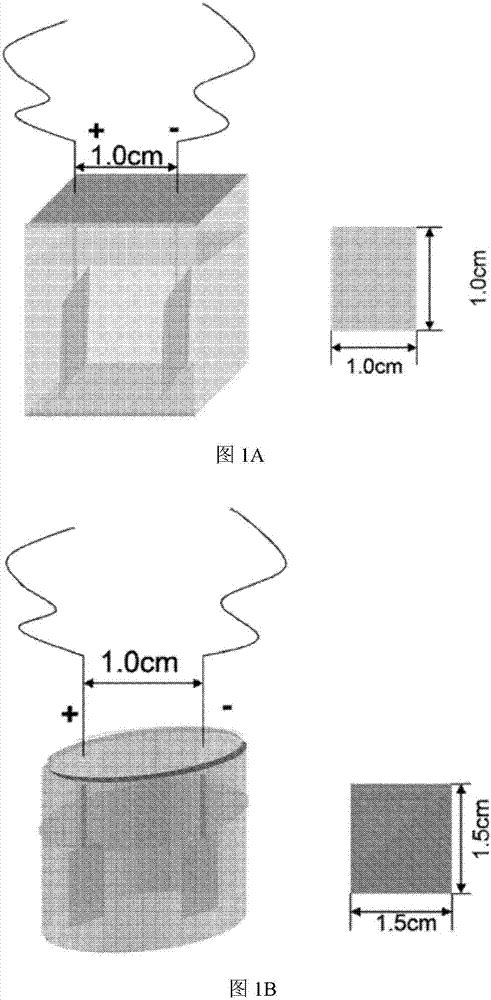
[0156] Rosiglitazone (10mg) was dissolved in 0.3M Et 4 NF·4HF in acetonitrile solution (10 mL). Transfer the solution to ECF pool. Using Ni electrodes, each reaction surface area is ~2.5cm 2 . The current (I) was set at 0.05A. The electrochemical reaction was performed at ambient temperature. The reaction was monitored by LC / MS (MW of product = 18 + MW of rosiglitazone).
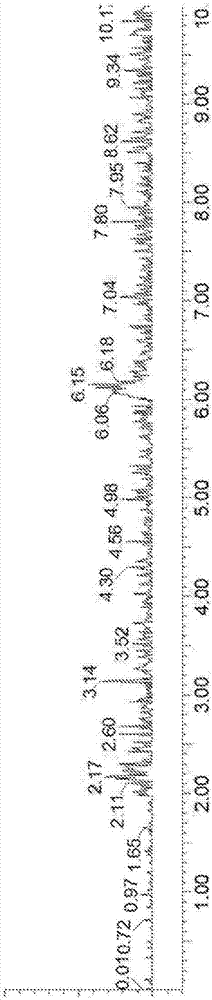
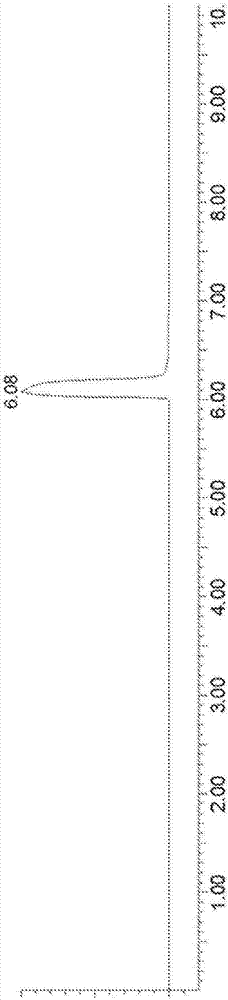
[0157] Figure 2A with Figure 2B Exemplary LC-MS chromatograms of the reaction mixture with MS detectors set to (18 + H + molecular weight (MW) of rosiglitazone) and (H + MW of rosiglitazone) are shown, respectively, before the reaction starts ). Figure 2C with Figure 2D An exemplary LC chromatogram of the reaction mixture is shown before the reaction starts, where the diode array detector (DAD) and MS detector detect the total ion current, respectively.
[0158] Figure 3A with Figure 3B Exemplary LC-MS chromato...
Embodiment 2
[0159] Embodiment 2: Electrochemical fluorination of telmisartan
[0160]
[0161] Telmisartan (10mg) was dissolved in 0.3M Et 4 NF·4HF in acetonitrile solution (10 mL). Transfer the solution to ECF pool. Using Pt electrodes, each reaction surface area is ~2.5cm 2 . The current (I) was set to 0.01A. Electrochemical reactions were performed at ambient temperature and monitored by LC / MS (MW of product = 18 + MW of starting material).
[0162] Figure 4A with Figure 4B Exemplary LC-MS chromatograms of the reaction mixture are shown, with the MS detector set to (18 + H + MW of Telmisartan) and (H + MW of Telmisartan), respectively, before the start of the reaction. Figure 4C with Figure 4D An exemplary LC chromatogram of the reaction mixture is shown before the reaction starts, where the diode array detector (DAD) and MS detector detect the total ion current, respectively.
[0163] Figure 5A with Figure 5B Exemplary LC-MS chromatograms of the reaction mixture ...
Embodiment 3
[0164] Example 3: Electrochemical fluorination of Sorafenib
[0165]
[0166] Sorafenib (5mg) was dissolved in 0.3M Et 4 NF·4HF in acetonitrile solution (10 mL). Transfer the solution to ECF pool. Using Ni electrodes, each reaction surface area is ~2.5cm 2 . The current (I) was set to 0.06A. The electrochemical reaction was performed at ambient temperature. The reaction was monitored by LC / MS (MW of product = 18 + MW of starting material).
[0167] Figure 6A with Figure 6B Exemplary LC-MS chromatograms of the reaction mixture with MS detector set to (18 + H + MW of sorafenib) and (H + MW of sorafenib), respectively, are shown before the reaction starts. Figure 6C with Figure 6D An exemplary LC chromatogram of the reaction mixture is shown before the reaction starts, where the diode array detector (DAD) and MS detector detect the total ion current, respectively.
[0168] Figure 7A with Figure 7B 10 hours after the start of the reaction, an exemplary LC-MS...
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com