Method for extracting aluminum sulfate, aluminum potassium sulfate, rubidium sulfate and cesium sulfate by aid of leaching agents of lithium mica sulfuric acid
A technology of aluminum potassium sulfate and lepidolite, which is applied in the fields of aluminum sulfate, chemical instruments and methods, rubidium/cesium/francium compounds, etc., and can solve problems such as extraction of aluminum sulfate and cesium sulfate that have not yet been discovered
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
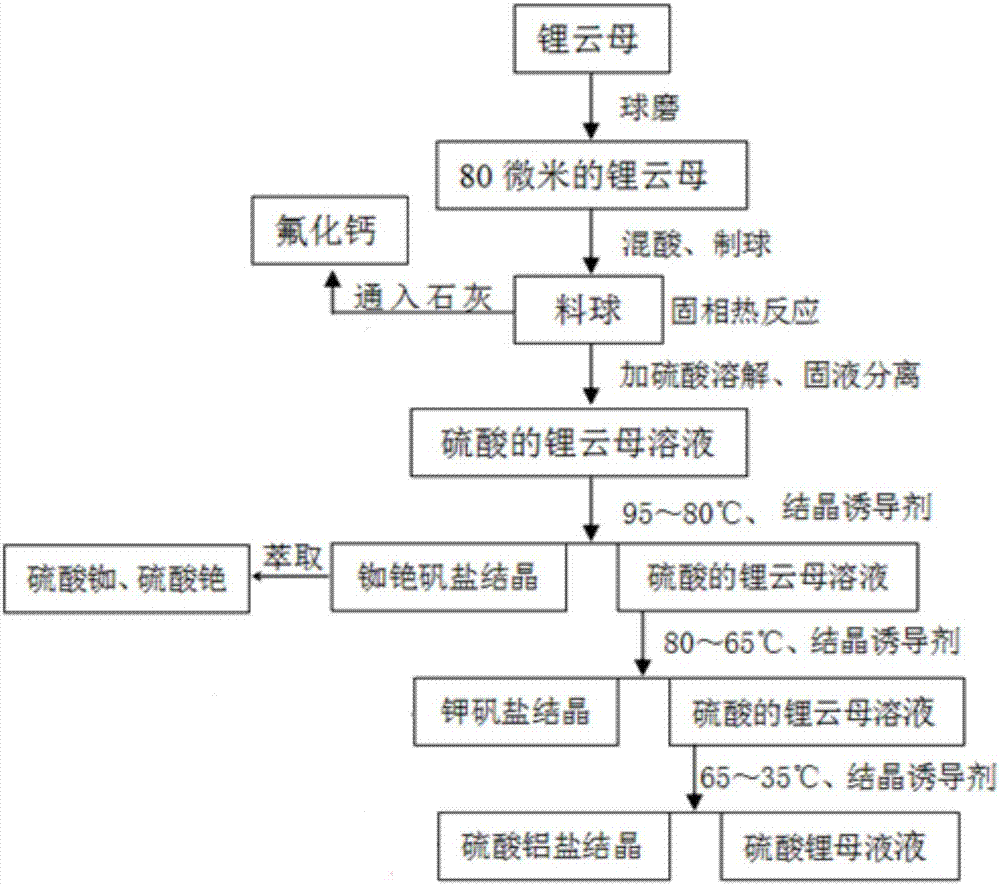
[0035] Such as figure 1 As shown, the method for extracting aluminum sulfate, aluminum potassium sulfate, rubidium sulfate and cesium sulfate using lepidolite sulfuric acid leaching solution provided in this embodiment comprises the following steps:
[0036] (1) Ball milling activation of lepidolite
[0037] The lepidolite is ball-milled to 200 mesh, and the iron is removed by an iron remover to obtain lepidolite powder for subsequent use;
[0038] (2) Sulfuric acid solid-phase thermal reaction
[0039] Mix the lepidolite powder obtained in step (1) with concentrated sulfuric acid solution with a mass concentration of 95% to obtain a mixture, then make the mixture into balls to obtain pellets, then heat the pellets to 300°C, and keep the Insulation reaction for 6 hours, the gas generated during the heating process is passed into lime water;
[0040] Among them, the mass ratio of lepidolite powder to concentrated sulfuric acid solution with a mass concentration of 95% is 1.2...
Embodiment 2
[0051] The method for extracting aluminum sulfate, potassium aluminum sulfate, rubidium sulfate and cesium sulfate by utilizing lepidolite sulfuric acid leaching solution provided in this embodiment comprises the following steps:
[0052] (1) Ball milling activation of lepidolite
[0053] The lepidolite is ball-milled to 100 mesh, and iron is removed by an iron remover to obtain lepidolite powder for subsequent use;
[0054] (2) Sulfuric acid solid-phase thermal reaction
[0055] Mix the lepidolite powder obtained in step (1) with concentrated sulfuric acid solution with a mass concentration of 97% to obtain a mixture, then make the mixture into balls to obtain pellets, then heat the pellets to 350°C, and keep Insulation reaction for 6 hours, the gas generated during the heating process is passed into lime water;
[0056] Among them, the mass ratio of lepidolite powder to concentrated sulfuric acid solution with a mass concentration of 97% is 1.1:1;
[0057] (3) Dilute sulf...
Embodiment 3
[0067] The method for extracting aluminum sulfate, potassium aluminum sulfate, rubidium sulfate and cesium sulfate by utilizing lepidolite sulfuric acid leaching solution provided in this embodiment comprises the following steps:
[0068] (1) Ball milling activation of lepidolite
[0069] The lepidolite is ball-milled to 400 mesh, and the iron is removed by an iron remover to obtain lepidolite powder for subsequent use;
[0070] (2) Sulfuric acid solid-phase thermal reaction
[0071] Mix the lepidolite powder obtained in step (1) with concentrated sulfuric acid solution with a mass concentration of 92% to obtain a mixture, then make the mixture into balls to obtain pellets, then heat the pellets to 400°C, and keep the Heat preservation reaction for 5 hours, and the gas generated during the heating process is passed into lime water;
[0072] Among them, the mass ratio of lepidolite powder to concentrated sulfuric acid solution with a mass concentration of 92% is 1.05:1;
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com